A) s
B) sp
C) sp2
D) sp3
Correct Answer

verified
Correct Answer
verified
True/False
A carbanion contains a carbon atom with a formal negative charge.
Correct Answer

verified
Correct Answer
verified
True/False
To form a ó bond,two atomic orbitals overlap to form a single molecular orbital.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the geometry around an sp hybridized carbon?
A) linear
B) trigonal planar
C) tetrahedral
D) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Figure 2
The following questions refer to the structure of heroin (shown below) . 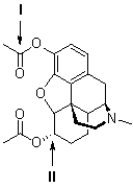 -Referring to Figure 2,what is the hybridization of the carbon atom at II?
-Referring to Figure 2,what is the hybridization of the carbon atom at II?
A) p
B) sp
C) sp2
D) sp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Figure 3
The following questions refer to the molecule drawn below. 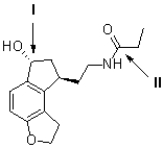 -Referring to Figure 3,how many sp2 atoms are contained in the molecule shown?
-Referring to Figure 3,how many sp2 atoms are contained in the molecule shown?
A) 6
B) 7
C) 8
D) 9
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Figure 2
The following questions refer to the structure of heroin (shown below) .  -Referring to Figure 2,what is the geometry of the carbon atom shown at II?
-Referring to Figure 2,what is the geometry of the carbon atom shown at II?
A) bent
B) trigonal planar
C) tetrahedral
D) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many orientations exist for a sp3 orbital?
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
True/False
A ó molecular orbital contains out-of-phase overlap of atomic orbitals.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many bonded and non-bonded electrons does a molecule with no formal charges and the formula C2H4O2 contain?
A) 4 bonded,4 non-bonded
B) 7 bonded.4 non-bonded
C) 7 bonded,5 non-bonded
D) 4 bonded,7 non-bonded
Correct Answer

verified
Correct Answer
verified
True/False
The two ð bonds in an triple bond are 180º from each other.
Correct Answer

verified
Correct Answer
verified
Essay
Assign non-zero formal charges to the following molecule. 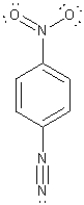
Correct Answer

verified
The charge...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
How many bonds does oxygen make while remaining neutral?
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many hydrogens does the following line structure contain? 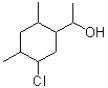
A) 1
B) 10
C) 19
D) 26
Correct Answer

verified
Correct Answer
verified
True/False
Only filled molecular orbitals contribute to bonding.
Correct Answer

verified
Correct Answer
verified
True/False
Empty p orbitals are incapable of contributing to resonance structures.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents the ground state electron configuration for a O2- ion?
A) 1s22s22p4
B) 1s22s22p2
C) 1s22s22p6
D) 2s22p4
Correct Answer

verified
Correct Answer
verified
True/False
In a O-H bond,the electron density is skewed towards the hydrogen atom.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Figure 2
The following questions refer to the structure of heroin (shown below) .  -Referring to Figure 2,what is the geometry of the carbon atom shown at I?
-Referring to Figure 2,what is the geometry of the carbon atom shown at I?
A) bent
B) trigonal planar
C) tetrahedral
D) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What best describes a wedged bond?
A) It looks like ![]() and represents going into the page.
and represents going into the page.
B) It looks like ![]() and represents going out of the page.
and represents going out of the page.
C) It looks like ![]() and represents going into the page.
and represents going into the page.
D) It looks like
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 84
Related Exams