A) I and II
B) II and III
C) I and III
D) III and IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following reaction,use the identity of the alkyl halide and nucleophile to determine which substitution mechanism occurs.Then determine which solvent affords the faster reaction. 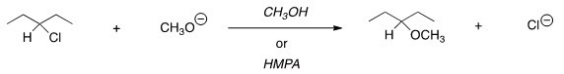
A) SN1,CH3OH
B) SN1,HMPA
C) SN2,CH3OH
D) SN2,HMPA
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 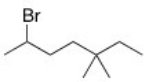
A) 2-Bromo-5,5-dimethylheptane
B) 3,3-Dimethyl-6-bromoheptane
C) 6-Bromo-3,3-dimethylheptane
D) 2-Bromo-5,5-dimethyloctane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction of tert-butyl bromide,(CH3) 3CBr,with ethanol affords the substitution product tert-butyl ethyl ether,(CH3) 3COCH2CH3,in acidic conditions.What would happen to the rate of the reaction if the concentration of ethanol was doubled?
A) The rate remains the same.
B) The rate decreases by a factor of 2.
C) The rate increases by a factor of 2.
D) The rate increases by a factor of 4.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction of what nucleophile and substrate is represented by the following transition state? 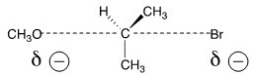
A) Methanol with 2-bromopropane
B) Methoxide with 2-bromopropane
C) Methoxide with 1-bromopropane
D) Methanol with 1-bromopropane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the rate-determining step of an SN1 reaction mechanism?
A) Reaction of the nucleophile with the carbocation to form the product.
B) Breaking the bond between the carbon and the leaving group to generate a carbocation.
C) Attack of the nucleophile on the substrate to generate a pentavalent carbon.
D) None of these.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkyl halides undergoes the fastest SN2 reaction with sodium hydroxide?
A) 1-Iodobutane
B) 1-Chlorobutane
C) 1-Fluorobutane
D) 1-Bromobutane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the Hammond postulate is true?
A) In an exothermic reaction,lowering the energy of the transition state increases the activation energy,Ea.
B) In an endothermic reaction,the more stable product forms faster.
C) In an endothermic reaction,the less stable product forms faster.
D) In an endothermic reaction,the activation energy,Ea,is similar for both products.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkyl halides is a vinyl alkyl halide? 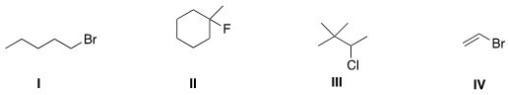
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 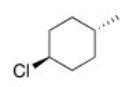
A) trans-4-Methylcyclohexyl chloride
B) trans-p-Chloromethylcyclohexane
C) trans-4-Methyl-1-chlorocyclohexane
D) trans-1-Chloro-4-methylcyclohexane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following reaction,use the identity of the alkyl halide and nucleophile to determine which substitution mechanism occurs.Then determine which solvent affords the faster reaction. 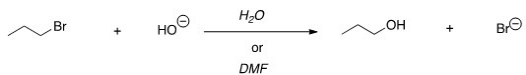
A) SN1,H2O
B) SN1,DMF
C) SN2,H2O
D) SN2,DMF
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction of 1-bromobutane with sodium hydroxide affords the substitution product 1-butanol.What would happen to the rate of the reaction if the concentration of both 1-bromobutane and sodium hydroxide were doubled?
A) The rate remains the same.
B) The rate decreases by a factor of 2.
C) The rate increases by a factor of 2.
D) The rate increases by a factor of 4.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about carbocation stability is true?
A) Carbocations are stabilized by electron-withdrawing inductive effects only.
B) Carbocations are stabilized by electron-withdrawing inductive effects and hyperconjugation.
C) Carbocations are stabilized by electron-donating inductive effects only.
D) Carbocations are stabilized by electro-donating inductive effects and hyperconjugation.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order of increasing SN2 reactivity? 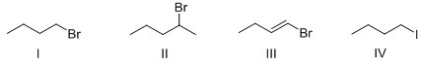
A) I > II > III > IV
B) IV > I > II > III
C) IV > III > II > I
D) III > II > I > IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkyl halides is a tertiary alkyl halide? 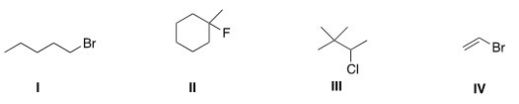
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following rate laws describes the kinetics of an SN1 reaction?
A) Rate = k[alkyl halide]
B) Rate = k[alkyl halide][nucleophile]
C) Rate = k[nucleophile]
D) Rate = k[solvent]
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures have the correct common name? 
A) I and II
B) II and III
C) II and IV
D) III and IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 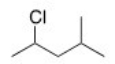
A) 2-Methyl-4-chloropentane
B) 2-Chloro-4-methylpentane
C) 2-Chloro-1-isopropylpropane
D) 2-Chloro-2-methylpentane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a polar aprotic solvent? 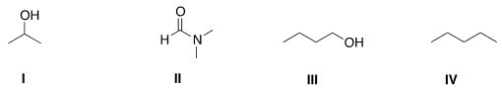
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the nucleophilic substitution reaction shown below? 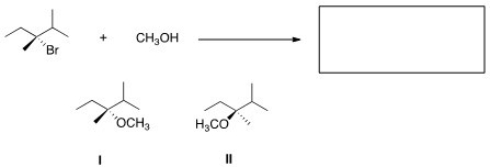
A) Only I
B) Only II
C) I and II
D) None
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 73
Related Exams