A) Outer electrons efficiently shield one another from nuclear charge.
B) Core electrons effectively shield outer electrons from nuclear charge.
C) Valence electrons are the most difficult of all electrons to remove.
D) Core electrons have the lowest ionization energies of all electrons.
E) Valence electrons in the outermost shell of all elements have the highest ionization energy.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An element that has the same ground state valence-shell electron configuration as thallium is
A) gallium.
B) carbon.
C) krypton.
D) cesium.
E) magnesium.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following orbital occupancy designations is incorrect?
A) 2s2
B) 3d6
C) 1s2
D) 4p3
E) 3d12
Correct Answer

verified
Correct Answer
verified
Essay
Explain the difference between paramagnetic and ferromagnetic materials.
Correct Answer

verified
Elements with unpaired electrons are par...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following orbital diagrams represents a paramagnetic atom? 1s 2s 2p
1.
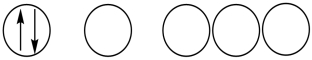 2.
2. 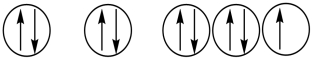 3.
3. 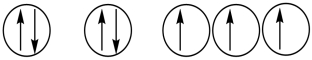
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 2 and 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is true of atomic radii?
A) They decrease down a group and remain constant across a period.
B) They decrease down a group and increase across a period.
C) They increase down a group and increase across a period.
D) They increase down a group and remain constant across a period.
E) They increase down a group and decrease across a period.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species has the largest radius?
A) Cl-
B) P
C) K-
D) Br-
E) Ca2+
Correct Answer

verified
Correct Answer
verified
Essay
Explain why the first ionization energy for oxygen is lower than that for nitrogen.
Correct Answer

verified
Oxygen has a 1s22s22p4 electron configurati...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
For which of the following atoms is the 2+ ion diamagnetic in the ground state?
A) Ni
B) Fe
C) Zn
D) Mn
E) Cu
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is a possible set of quantum numbers for an unpaired electron in the orbital box diagram below? 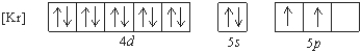
A) n = 1, = 1, = -1, ms = +1/2
B) n = 4, = 2, = -1, ms = -1/2
C) n = 5, = 2, = -2, ms = +1/2
D) n = 5, = 0, = 0, ms = -1/2
E) n = 5, = 1, = -1, ms = +1/2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the maximum number of electrons that can occupy one s orbital?
A) 1
B) 2
C) 6
D) 10
E) 14
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the ground state electron configuration of an element is [Ar]3d104s24p5, what is the typical charge on the monatomic anion of the element?
A) 4+
B) 2+
C) 1-
D) 2-
E) 3-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element has the following ground state electron configuration? 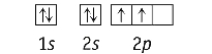
A) Be
B) O
C) Li
D) Si
E) N
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground state electron configuration for Sn2+?
A) [Kr]4d105s2
B) [Kr]4d105p2
C) [Kr]5s2
D) [Kr]4d105s25p2
E) [Kr]4d105s25p4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The change in energy for which of the following processes corresponds to the first ionization energy of beryllium?
A) Be(g) → Be+(g) + e-
B) Be(s) → Be+(s) + e-
C) Be(s) → Be+(g) + e-
D) Be(g) → Be2+(g) + 2e-
E) Be(s) + e- → Be-(s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electron configuration of the chloride ion?
A)
B)
C)
D)
E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
_____ have no affinity for electrons.
A) Transition metals
B) s-block elements
C) Main group nonmetals
D) Noble gases
E) Semiconductors
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The procedure by which electrons are assigned to (or built up into) orbitals is known as the ____ principle.
A) Aufbau
B) Bohr
C) Planck
D) Hund
E) Pauli
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many valence electrons does an arsenic atom have?
A) 5
B) 8
C) 7
D) 2
E) 33
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the correct valence shell orbital box notation for the ground state electron configuration of Fe?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 80
Related Exams