A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) All of these reactions probably release gamma radiation
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Radioactive isotopes are
A) very stable isotopes.
B) highly chemically reactive.
C) unstable isotopes.
D) charged species.
E) unusually nonreactive.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following choices describes an acute dose of radiation?
A) the dose that an x-ray technician receives over the course of her career
B) the dose that a scientist receives while working with radioactive materials for a multiyear research project
C) the annual dose that a pilot is expose to
D) the dose that is received by handling a highly radioactive source
E) the annual dose that we all receive as a result of background radiation
Correct Answer

verified
Correct Answer
verified
Multiple Choice
_____ have a negative charge.
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atom is in its ground state? 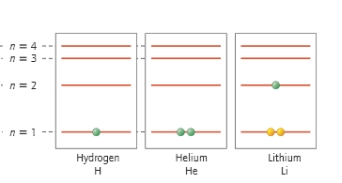
A) H
B) He
C) H and He
D) Li
E) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Micro- and macronutrients are
A) equally distributed throughout the body.
B) all metals and metalloids.
C) obtained through the diet.
D) only found in the first three periods of the periodic table.
E) all required in quantities of more than 100 mg per day.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about isotopes is false?
A) Isotopes are atoms with same number of protons but different numbers of neutrons.
B) Most elements naturally have more than one isotope.
C) Isotopes are atoms with the same atomic number but different mass numbers.
D) An isotope with more neutrons will have a greater mass than an isotope with fewer neutrons.
E) All of the above are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the current model of the atom, the part of the diagram labeled A is made up of 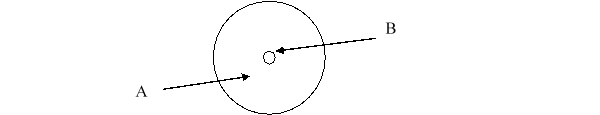
A) protons.
B) neutrons.
C) electrons.
D) protons and neutrons.
E) protons, neutrons, and electrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are in the valence shell of the ion represented by the energy diagram shown below? 
A) 8
B) 10
C) 12
D) 22
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Oxygen is a _______ and therefore _______ when it forms an ion.
A) metal; loses electrons
B) metal; gains electrons
C) nonmetal; loses electrons
D) nonmetal; gains electrons
E) metalloid; loses electrons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the atomic number of element X? 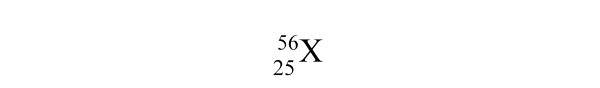
A) 25
B) 56
C) 81
D) 31
E) None of the above values is the atomic number.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements has a mass number of 35 and an atomic number of 17?
A) chlorine
B) bromine
C) argon
D) tellurium
E) sulfur-35
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of protons is equal to the _____ in a neutral atom.
A) number of neutrons
B) number of electrons
C) mass number
D) average atomic mass
E) group number
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT a building block element?
A) C
B) H
C) O
D) N
E) These are all building block elements.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The most common nutritional deficiency in the world is_____.
A) scurvy
B) anemia
C) kwashiorkor
D) rickets
E) pellagra
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following indicates that an alpha particle has been released during radioactive decay of an atom?
A) The identity of the atom does not change.
B) The mass number of the atom decreases by 4.
C) The atomic number of the atom increases by 1
D) The atomic number of the atom decreases by 1.
E) The atomic number of the atom decreases by 4.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nonmetals typically ______ and metals typically ______.
A) donate electrons; accept electrons
B) accept electrons; donate electrons
C) donate protons; accept protons
D) accept protons; donate protons
E) donate neutrons; accept neutrons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following groups of elements forms anions?
A) noble gases
B) transition metals
C) alkaline metals
D) alkali earth metals
E) halogens
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT a symptom of iron deficiency?
A) dizziness
B) shortness of breath
C) frequent urination
D) headaches
E) fatigue
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How is a beta particle different from an electron?
A) They are the same.
B) A beta particle is higher energy than a regular electron
C) A regular electron is higher energy than a beta particle.
D) A beta particle is positively charged.
E) A beta particle is positively charged and higher in energy than a regular electron.
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 135
Related Exams