A) Keq = 0.025
B) Keq = 5.2
C) Keq = 8.4 x 10-5
D) Keq = 6.3 x 105
E) not enough information
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
In a chemical reaction at constant temperature, the addition of a catalyst
A) affects the equilibrium constant.
B) increases the average kinetic energy of the reactants.
C) provides an alternate reaction pathway with a different activation energy.
D) decreases the energy released in the chemical reaction.
E) increases the concentration of the products at equilibrium.
Correct Answer

verified
C
Correct Answer
verified
True/False
When square brackets are placed around the formula for a substance in an equilibrium constant expression, it indicates the percent-by-mass concentration of that substance.For example [NaOH] means the percent-by-mass concentration of NaOH.
Correct Answer

verified
Correct Answer
verified
True/False
If heat is added to the system of an endothermic reaction, the equilibrium will shift to make more reactants.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements regarding catalysts is incorrect?
A) A catalyst increases the rate of a reaction by giving the reaction an alternate pathway with lower activation energy.
B) A catalyst is formed temporarily in an early step of a reaction.
C) Most enzyme catalysts are large protein molecules.
D) In a chemical equation the catalyst is shown above the reaction arrow of the equation.
E) The shape of an active site on an enzyme is unique, allowing it to react with only one substrate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which equilibrium constant represents a reaction that is product favored?
A) Keq = 0.025
B) Keq = 5.2
C) Keq = 8.4 x 10-5
D) Keq = 6.3 x 105
E) not enough information
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate of a reaction can be increased by all of the following except
A) increasing the temperature.
B) increasing the concentration of the reactants.
C) increasing the surface area of the reactants.
D) adding a catalyst.
E) increasing the volume of the reaction vessel.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction carried out in a sealed container: N2(g) + 3H2(g) ⇌ 2NH3(g) A state of equilibrium can be reached if the container initially contains
A) NH3 only.
B) N2 and H2 only.
C) NH3 and N2 only.
D) NH3, N2, and H2.
E) any of these combinations of reactants and product.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In order to write the equilibrium constant expression for a reaction, you must:
A) know the mechanism for the reaction.
B) know the rate of the forward and reverse reactions.
C) know the concentrations of all reactants and products.
D) have the balanced chemical equation for the reaction.
E) know the conditions of pressure, temperature, and concentration for the system.
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Identify any intermediates or catalysts in the following two-step reaction: Cu2+ + H2 CuH+ + H+ CuH+ + H+ + H2C=CH2 Cu2+ + H3C-CH3
A) CuH+ is an intermediate.
B) Cu2+ is a catalyst, H+ is an intermediate.
C) Cu2+ is a catalyst, CuH+ is an intermediate.
D) Cu2+ is a catalyst, CuH+ and H+ are intermediates.
E) CuH+ and H+ are catalysts, Cu2+ is an intermediate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given that the following reaction is exothermic, which of the following sets of changes would cause the following system at equilibrium to shift to increase the number of N2O4(g) molecules? 2NO2(g) ⇌N2O4(g) What observation would indicate that the reaction is at equilibrium in a closed container?
A) Increasing container volume and increasing temperature
B) Increasing container volume and decreasing temperature
C) Decreasing container volume and increasing temperature
D) Decreasing container volume and decreasing temperature
E) Changing volume or temperature will not affect the number of gas molecules present at equilibrium
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify an intermediate in the following two-step reaction.Sn2+ + Fe3+ Sn3+ + Fe2+ Sn3+ + Fe3+ Sn4+ + Fe2+
A) Sn2+
B) Fe3+
C) Sn3+
D) Fe2+
E) Sn4+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify any intermediates or catalysts in the following two-step reaction: H2O2 + 2Br- + 2H+ 2H2O + Br2 H2O2 + Br2 2H+ + O2 + 2Br-
A) H2O2 is an intermediate.
B) H2O2 is a catalyst, Br- is an intermediate.
C) Br- is a catalyst, Br2 is an intermediate.
D) H+ is a catalyst.
E) Br- and H+ are catalysts, Br2 is an intermediate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the catalyst in the following three-step process for the decomposition of ozone.O3(g) ⇌ O2(g) + O(g) NO(g) + O3(g) NO2(g) + O2(g) O(g) + NO2(g) O2(g) + NO(g)
A) O(g)
B) O3(g)
C) O2(g)
D) NO(g)
E) NO2(g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements regarding catalysts is incorrect?
A) A catalyst increases the rate of a reaction by giving the reaction an alternate pathway with a lower activation energy.
B) Catalysts need not be present in large amounts because they are regenerated during the reaction.
C) Enzymes act as catalysts in our bodies.
D) In a chemical reaction, the catalyst is shown on the reactant side of the equation.
E) The shape of an active site on an enzyme is unique, allowing it to react with only one substrate.
Correct Answer

verified
Correct Answer
verified
True/False
A reaction that has a high activation energy will have a high rate of reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction CO(g) + H2O(g) CO2(g) + H2(g) , represented by the following diagram: 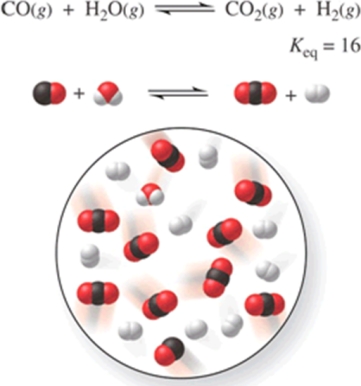 What is the composition of this system when the reaction reaches equilibrium?
What is the composition of this system when the reaction reaches equilibrium?
A) 3 CO, 3 H2O, 7 CO2, 7 H2
B) 1 CO, 1 H2O, 4 CO2, 4 H2
C) 2 CO, 2 H2O, 8 CO2, 8 H2
D) 8 CO, 8 H2O, 2 CO2, 2 H2
E) 4 CO, 6 H2O, 4 CO2, 6 H2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify any intermediates or catalysts in the following two-step reaction: S2O82- + I- 2SO42- + I+ I+ + I- I2
A) S2O82- is an intermediate.
B) S2O82- is a catalyst.
C) I- is a catalyst.
D) I+ is an intermediate.
E) SO42- is a catalyst.
Correct Answer

verified
Correct Answer
verified
True/False
A catalyst gives an alternate pathway for a reaction that has a lower activation energy.
Correct Answer

verified
Correct Answer
verified
True/False
The reaction CaCO3(s)  CaO(s) + CO2(g) is an example of a heterogeneous equilibrium.
CaO(s) + CO2(g) is an example of a heterogeneous equilibrium.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 52
Related Exams