A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of the answers is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Polonium-208 is an alpha emitter with a half-life of 2.90 years. How many milligrams of polonium from an original sample of 2.00 mg will remain after 8.00 years?
A) 0.147 mg
B) 0.296 mg
C) 0.725 mg
D) 6.77 mg
E) 1.90 mg
Correct Answer

verified
Correct Answer
verified
Short Answer
Electron capture is the capture of an electron-usually a 1s electron-by the ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which isotope, when bombarded with bismuth-209, would yield two neutrons and an isotope with atomic number 121 and mass number 299?
A) Pb-211
B) Po-209
C) Sr-92
D) Rn-38
E) Sr-38
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name for spontaneous emission of particles or electromagnetic radiation by certain nuclei?
A) Protons
B) Isotopes
C) Radioactivity
D) Neutrons
E) Electrons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
It is believed that two carbon-12 nuclei can react in the core of a supergiant star to form sodium-23 and hydrogen-1. Calculate the energy released from this reaction for each mole of hydrogen formed. 
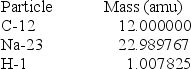 (1 kg = 6.022 × 1026 amu; NA = 6.022 × 1023 mol-1;
C = 2.99792458 × 108 m/s)
(1 kg = 6.022 × 1026 amu; NA = 6.022 × 1023 mol-1;
C = 2.99792458 × 108 m/s)
A) 2.16 × 1014 kJ
B) 2.16 × 1011 kJ
C) 2.16 × 108 kJ
D) 2.16 × 105 kJ
E) None of the answers is correct.
Correct Answer

verified
C
Correct Answer
verified
True/False
For stable atoms of elements having low atomic numbers (≤ 20), the neutron-to-proton ratio is close to zero.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Present-day plant life has a carbon-14 decay rate of 16 disintegrations per minute (dpm) per gram of carbon. If a contemporary wooden chair were preserved for the next 3900 years, what 14C decay rate should be expected from the wood used to make the chair? (t1/2 = 5730 yr)
A) 26 dpm
B) 12 dpm
C) 11 dpm
D) 10 dpm
E) 8 dpm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Iodine-131, t1/2 = 8.0 days, is used in the diagnosis and treatment of thyroid gland diseases. If a laboratory sample of iodine-131 initially emits 9.95 × 1018 β particles per day, how long will it take for the activity to drop to 6.22 × 1017 β particles per day?
A) 2.0 days
B) 16 days
C) 32 days
D) 128 days
E) None of the answers is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The carbon-14 activity of some ancient Indian corn was found to be 7.0 disintegrations per minute (dpm) per gram of carbon. If present-day plant life has 16 dpm per gram of carbon, how old is the Indian corn? (t1/2 = 5730 yr)
A) 6800 yr
B) 2500 yr
C) 4700 yr
D) 10,000 yr
E) 7200 yr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the nuclear process called in which a heavy nuclear of mass number greater than 200 divides to form smaller nuclei of intermediate mass and one or more neutron(s) ?
A) Photonuclear reactions
B) Nuclear fission
C) Thermal conductivity
D) Nuclear combination
E) Nuclear fusion
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cobalt-60 is a beta emitter with a half-life of 5.3 years. Approximately what fraction of cobalt-60 atoms will remain in a particular sample after 26.5 years?
A) 1/5
B) 1/16
C) 1/26
D) 1/32
E) 1/64
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following diagrams, A represents a radioactive isotope that decays into a new isotope, X. Each circle represents 1 mmol of atoms. t = 0: 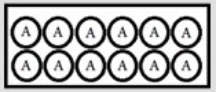 t = 25.0 s:
t = 25.0 s: 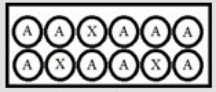 Over the first 25.0 s, what is the disintegration rate of A?
Over the first 25.0 s, what is the disintegration rate of A?
A) 0.120 disintegrations per second
B) 1.81 × 1021 disintegrations per second
C) 3.00 disintegrations per second
D) 7.23 × 1019 disintegrations per second
E) 1.38 × 10-20 disintegrations per second
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sodium-21 will emit positrons, each having an energy of 4.0 × 10-13 J. What is this energy in MeV? (1 MeV = 1.602 × 10-13 J)
A) 4.0 × 10-7 MeV
B) 2.5 MeV
C) 40 MeV
D) 2.5 × 106 MeV
E) 2.5 × 10-6 MeV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is an incorrect representation of the indicated particle or nucleus?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the name for the minimum mass of fissionable material required to generate a self-sustaining nuclear chain reaction?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In passing through matter, alpha particles lose energy chiefly by causing
A) fermentation.
B) neutralization.
C) ionization.
D) condensation.
E) carbonation.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the nuclear binding energy per nucleon for 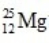 ? (1 kg = 6.022 × 1026 amu;
C = 2.99792458 × 108 m/s)
Particle Mass (amu)
? (1 kg = 6.022 × 1026 amu;
C = 2.99792458 × 108 m/s)
Particle Mass (amu) 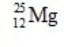 24.985839
24.985839 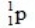 1.007276
1.007276 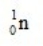 1.008665
1.008665
A) 0.214 J/nucleon
B) 3.20 × 10-11 J/nucleon
C) 1.28 × 10-12 J/nucleon
D) 0.999 J/nucleon
E) 7.35 × 10-11 J/nucleon
Correct Answer

verified
Correct Answer
verified
True/False
Gamma rays are high energy electrons.
Correct Answer

verified
False
Correct Answer
verified
Multiple Choice
Which of the following is injected into the bloodstream to trace the flow of blood and detect possible constrictions or obstructions in the circulatory system?
A) (18O)
B) (131I)
C) (123I)
D) (24Na)
E) (99Tc)
Correct Answer

verified
D
Correct Answer
verified
Showing 1 - 20 of 127
Related Exams