A) Stanley Miller
B) Jakob Berzelius
C) Friedrich Wohler
D) Hermann Kolbe
E) August Kekulé
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Research indicates that Albuterol, a drug used to relax bronchial muscles, improving airflow and thus offering relief from asthma, consists only of one enantiomer, the R-form. Why is it important for this drug to consist of only one enantiomeric form, rather than a mixture of enantiomers?
A) Different enantiomers may have different or opposite physiological effects.
B) It is impossible to synthesize mixtures of enantiomers.
C) It is much less expensive to synthesize one enantiomer at a time.
D) Albuterol is an example of a compound for which only one enantiomer exists.
E) Only the R-form of Albuterol has been studied; until more information is available, physicians prefer to use the pure R-form.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Research indicates that Ibuprofen, a drug used to relieve inflammation and pain, is a mixture of two enantiomers; that is, molecules that
A) have identical three-dimensional shapes.
B) are mirror images of one another.
C) lack an asymmetric carbon.
D) differ in the location of their double bonds.
E) differ in their electrical charge.
Correct Answer

verified
B
Correct Answer
verified
Short Answer
The following questions refer to the functional groups shown in Figure 4.6.
A.
D.
B.
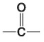 E. - SH
C.
E. - SH
C.
 -Which is a basic functional group that can accept H+ and become positively charged?
-Which is a basic functional group that can accept H+ and become positively charged?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A carbon skeleton is covalently bonded to both an amino group and a carboxyl group. When placed in water it
A) would function only as an acid because of the carboxyl group.
B) would function only as a base because of the amino group.
C) would function as neither an acid nor a base.
D) would function as both an acid and a base.
E) is impossible to determine how it would function.
Correct Answer

verified
Correct Answer
verified
Short Answer
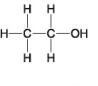
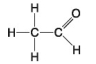
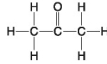
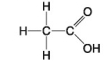
The following questions refer to the molecules shown in Figure 4.7.
A.
 B.
B.
 C.
C.
 D.
D.
 E.
E.
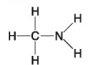 -Which molecule is an alcohol?
-Which molecule is an alcohol?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following people used this apparatus to study formation of organic compounds?
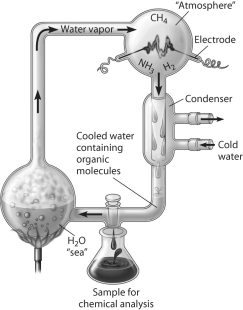
A) Stanley Miller
B) Jakob Berzelius
C) Friedrich Wohler
D) Hermann Kolbe
E) August Kekulé
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which two functional groups are always found in amino acids?
A) ketone and aldehyde
B) carbonyl and carboxyl
C) carboxyl and amino
D) phosphate and sulfhydryl
E) hydroxyl and aldehyde
Correct Answer

verified
Correct Answer
verified
Short Answer
The following questions refer to the molecules shown in Figure 4.7.
A.
 B.
B.
 C.
C.
 D.
D.
 E.
E.
 -Which molecule contains a carboxyl group?
-Which molecule contains a carboxyl group?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A carbon atom is most likely to form what kind of bond(s) with other atoms?
A) ionic
B) hydrogen
C) covalent
D) A and B only
E) A, B, and C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
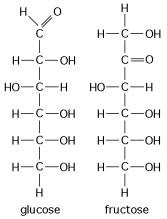 -Shown here in Figure 4.2 are the structures of glucose and fructose. These two molecules differ in the
-Shown here in Figure 4.2 are the structures of glucose and fructose. These two molecules differ in the
A) number of carbon, hydrogen, and oxygen atoms.
B) types of carbon, hydrogen, and oxygen atoms.
C) arrangement of carbon, hydrogen, and oxygen atoms.
D) number of oxygen atoms joined to carbon atoms by double covalent bonds.
E) answers A, B, and C
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
The experimental approach taken in current biological investigations presumes that
A) simple organic compounds can be synthesized in the laboratory from inorganic precursors, but complex organic compounds like carbohydrates and proteins can only be synthesized by living organisms.
B) a life force ultimately controls the activities of living organisms and this life force cannot be studied by physical or chemical methods.
C) although a life force, or vitalism, exists in living organisms, this life force cannot be studied by physical or chemical methods.
D) living organisms are composed of the same elements present in nonliving things, plus a few special trace elements found only in living organisms or their products.
E) living organisms can be understood in terms of the same physical and chemical laws that can be used to explain all natural phenomena.
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
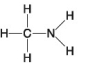
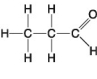
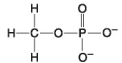
The following questions refer to the molecules shown in Figure 4.8.
A.
 B.
B.
 C.
C.
 D.
D.
 E.
E.
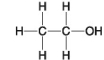 -Organic chemistry is currently defined as
-Organic chemistry is currently defined as
A) the study of compounds made only by living cells.
B) the study of carbon compounds.
C) the study of vital forces.
D) the study of natural (as opposed to synthetic) compounds.
E) the study of hydrocarbons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the pairs of molecular structures shown below do NOT depict enantiomers (enantiomeric forms) of the same molecule?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Short Answer
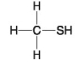
The following questions refer to the molecules shown in Figure 4.8.
A.
 B.
B.
 C.
C.
 D.
D.
 E.
E.
 -Which molecule contains a sulfhydryl functional group?
-Which molecule contains a sulfhydryl functional group?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements correctly describes geometric isomers?
A) They have variations in arrangement around a double bond.
B) They have an asymmetric carbon that makes them mirror images.
C) They have the same chemical properties.
D) They have different molecular formulas.
E) Their atoms and bonds are arranged in different sequences.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following people synthesized an organic compound, acetic acid, from inorganic substances that had been prepared directly from pure elements?
A) Stanley Miller
B) Jakob Berzelius
C) Friedrich Wohler
D) Hermann Kolbe
E) August Kekulé
Correct Answer

verified
Correct Answer
verified
Short Answer
The following questions refer to the functional groups shown in Figure 4.6.
A.
D.
B.
 E. - SH
C.
E. - SH
C.
 -Which is an amino functional group?
-Which is an amino functional group?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Three or four of the pairs of structures shown below depict enantiomers (enantiomeric forms) of the same molecule. Which pair, if any, are NOT enantiomers of a single molecule? If each of the pairs depicts enantiomers, choose answer F.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
F) Both illustrations in each of the other answer choices depict enantiomers of the same molecule.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One of the following people was the first to suggest that organic compounds, those found in living organisms, were distinctly different from inorganic compounds found in the nonliving world. Though this suggestion is now known to be incorrect, it stimulated important research into organic compounds. Who suggested this?
A) Stanley Miller
B) Jakob Berzelius
C) Friedrich Wohler
D) Hermann Kolbe
E) August Kekulé
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 68
Related Exams