A) The monomer has to have at least one double bond.
B) The monomer has to have at least two functional groups (though they can be the same) .
C) The monomer has to have at least two functional groups (though they must be the different) .
D) The monomer has to be very small.
E) The monomer has to have a number of polymer components.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A common inactive ingredient in products such as sunscreen lotions and shampoo is triethyl amine,also known as TEA.Which of the following is the chemical structure for this compound? 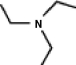
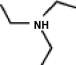
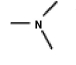
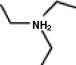 (a) (b) (c) (d)
(a) (b) (c) (d)
A) a
B) b
C) c
D) d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If champagne has a proof of 28,how many milliliters of alcohol are in 250 mL of champagne?
A) 35
B) 38
C) 28
D) 3.8
E) 200
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which would you expect to be more viscous,a polymer made of long molecular strands or one made of short molecular strands? Why?
A) short molecular strands because of a greater density
B) long molecular strands because they tend to tangle among themselves
C) short molecular strands because their ends are typically polar
D) long molecular strands because of a greater molecular mass
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following stick structures could describe pentane (C5H12) ? 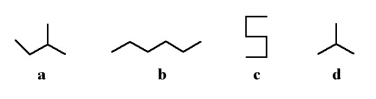
A) a
B) b
C) c
D) d
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following picture shows an organic molecule: 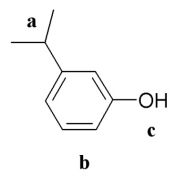 Which region (a-b-c) is a phenolic functional group?
Which region (a-b-c) is a phenolic functional group?
A) a
B) b
C) c
D) b or c
E) b and c
Correct Answer

verified
Correct Answer
verified
Multiple Choice
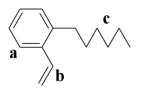 -Which of the above sections of this molecule would be considered to be the aromatic portion of the molecule?
-Which of the above sections of this molecule would be considered to be the aromatic portion of the molecule?
A) a
B) b
C) c
D) a and b
E) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a fractionating tower,the crude oil vapors pass from a pipe still into the column.Tar and lubricating stock are the first components to be pulled off at the bottom.Nearer the top kerosene is pulled off followed by gasoline and finally natural gas at the very top.From this information,which has a higher boiling point,gasoline or kerosine?
A) Gasoline has the higher boiling point.
B) Kerosene has the higher boiling point.
C) Their boiling points are the same,but kerosene has the greater density.
D) Fractional distillation components are pulled off based on molecular weight,so it is not possible to know which has the higher boiling point from the information given.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules could be a structural isomer for the underlined molecule below? 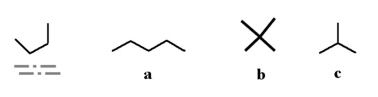
A) a
B) b
C) c
D) all of the above
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
 -Which of the above sections of this molecule would be considered a saturated portion of the molecule?
-Which of the above sections of this molecule would be considered a saturated portion of the molecule?
A) a
B) b
C) c
D) a and b
E) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
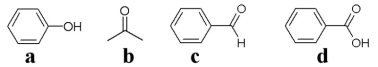 -Which of the above molecules contains a carbonyl group?
-Which of the above molecules contains a carbonyl group?
A) a
B) all but a
C) all but b
D) c
E) all but d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What determines the chemical and physical properties of hydrocarbons?
A) the way the atoms are connected together
B) the number of carbon and hydrogens
C) the elements it is composed of
D) the number of oxygen
E) both A and B
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ethers has the highest boiling point?
A) CH3OCH3
B) CH3CH2OCH2CH3
C) CH3CH2CH2OCH2CH2CH3
D) CH3CH2CH2CH2OCH2CH2CH2CH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would the formula be for the following stick model? 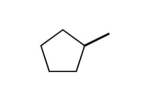
A) C6H12
B) C5H5
C) C6H10
D) C5H10
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements best describes the concept of structural conformation?
A) rearranging the shape of a molecule by rotating around bonds
B) rearranging the shape of a molecule by changing the arrangement of bonds
C) changing the structure by adding or subtracting bonds
D) arranging the atom into the best conformation for the given structure
E) changing the conformation to generate a new molecule with different chemical properties
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One solution to the problem of our overflowing landfills is to burn plastic objects instead of burying them.What would be some of the advantages and disadvantages of this practice?
A) disadvantage: toxic air pollutants; advantage: reduced landfill volume
B) disadvantage: loss of vital petroleum-based resource; advantage: generation of electricity
C) disadvantage: discourages recycling; advantage: provides new jobs
D) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Carbon-carbon single bonds can rotate while carbon-carbon double bonds cannot rotate.How many different structures are shown below. 
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is a hydrocarbon?
A) It is a molecule composed of carbon and hydrogen only.
B) It is a wet carbon atom.
C) It is any organic molecule.
D) It is a molecule derived from hydrogen synthesis.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an aromatic molecule? 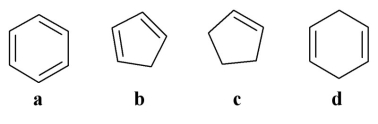
A) a
B) b
C) c
D) d
E) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the hallmark of a saturated hydrocarbon?
A) a hydrocarbon where carbon has only a single bond to two or more other carbons
B) a hydrocarbon that is completely dissolved in water
C) a hydrocarbon where carbon has multiple bonds to one or more other carbons
D) any molecule that is completely dissolved in a hydrocarbon solvent
E) none of the above
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 96
Related Exams