A) Atomic mass
B) Number of protons
C) Oxidation number
D) Lewis structure
E) None of the answers is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At a temperature of 27 K, neon condenses due to
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) covalent bonding.
E) intramolecular forces.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the most reasonable prediction for the H-N-H bond angle in NH3?
A) 90°
B) 109.5°
C) 120°
D) 107°
E) 105°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following atoms does not participate in hydrogen bonding?
A) S
B) O
C) F
D) N
E) H
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which substance should exhibit hydrogen bonding in the liquid phase?
A) PH3
B) He
C) H2S
D) CH4
E) CH3OH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the most reasonable prediction for the Cl-N-Cl bond angle in NCl3?
A) 120°
B) 111°
C) 109.5°
D) 107°
E) 90°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of NO2− as predicted by the VSEPR model?
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the most reasonable prediction for the H-C-H bond angle in CH4?
A) 90°
B) 109.5°
C) 120°
D) 107°
E) 105°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of NOCl as predicted by the VSEPR model? 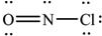
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct Lewis structure for NOCl, a reactive material used as an ionizing solvent.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the predicted molecular geometry of the H2O molecule according to the VSEPR model?
A) tetrahedral
B) trigonal pyramidal
C) bent
D) square planar
E) seesaw
Correct Answer

verified
Correct Answer
verified
Showing 61 - 71 of 71
Related Exams