Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds exhibits hydrogen bonding?
A) CH3Cl
B) HI
C) CH3OCH3
D) NH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which has the smallest dipole-dipole forces?
A) CH3F
B) HCl
C) N2
D) CO
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A supercritical fluid refers to a substance
A) above both its critical temperature and its critical pressure.
B) at its triple point.
C) that is in the liquid crystal state.
D) with a viscosity of zero.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which drawing below best represents hydrogen bonding methanol,CH3OH? 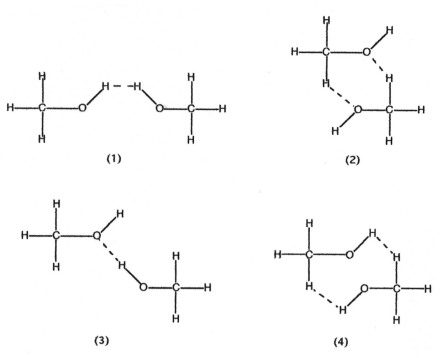
A) drawing (1)
B) drawing (2)
C) drawing (3)
D) drawing (4)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which best indicates the direction of the dipole moment in formaldehyde,H2C=O? 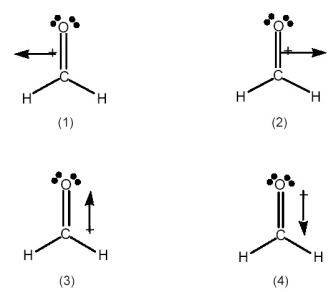
A) drawing (1)
B) drawing (2)
C) drawing (3)
D) drawing (4)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following exhibits ion-dipole forces?
A) NaCl(s)
B) NaCl(aq)
C) Na(s)
D) Cl2(g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Solids having no ordered long-range structure are classified as
A) amorphous
B) crystalline
C) metallic
D) molecular
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the physical phase of the substance at T = 225 K and P = 1.1 atm?
A) gas
B) liquid
C) solid
D) supercritical fluid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The critical temperature of a substance is the
A) highest temperature at which the liquid phase can exist in equilibrium with the gas phase.
B) temperature above which the compound decomposes.
C) temperature at which all three phases can exist in equilibrium.
D) temperature at which sublimation occurs.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The vapor pressure of liquid chloroform,CHCl3,is 400.0 torr at 24.1°C and 100.0 torr at -6.3°C.What is ΔHvap of chloroform?
A) 15.3 kJ/mol
B) 30.1 kJ/mol
C) 57.6 kJ/mol
D) 86.7 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules does not have a dipole moment?
A) C2H2
B) H2O
C) CH3CH2OH
D) H I
Correct Answer

verified
Correct Answer
verified
Multiple Choice
KCl crystallizes in a cubic unit cell with Cl- ions on each corner and each face.How many K+ ions and Cl- ions are in each unit cell of KCl?
A) 1 K+ ion and 1 Cl- ion
B) 2 K+ ions and 2 Cl- ions
C) 4 K+ ions and 4 Cl- ions
D) 8 K+ ions and 8 Cl- ions
Correct Answer

verified
Correct Answer
verified
Short Answer
The intermolecular forces formed when NaCl is dissolved in water are ________ forces.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the physical phase of the substance at T = 400 K and P = 2.0 atm?
A) gas
B) liquid
C) solid
D) supercritical fluid
Correct Answer

verified
Correct Answer
verified
Short Answer
The Br-Cl bond has 5.05% ionic character and a dipole moment of 0.518 D.What is the distance between atoms in BrCl?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The phase diagram of a substance is shown below. 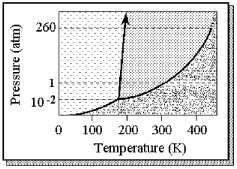 -The approximate normal melting point of this substance is
-The approximate normal melting point of this substance is
A) 100 K.
B) 190 K.
C) 300 K.
D) 430 K.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A certain mineral crystallizes in the cubic unit cell shown below.M represents the cations and A represents the anions.What is the empirical formula of the mineral? 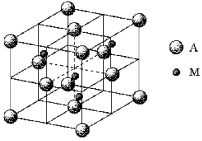
A) MA
B) MA2
C) M2A
D) M4A4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the process: HNO3(g) ⇌ HNO3(l) ΔH° is -39.04 kJ/mol and ΔS° is -111.74 J/(mol ∙ K) .What is the normal boiling point of pure HNO3?
A) 2) 86°C
B) 76.2°C
C) 270.3°C
D) 349.4°C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which drawing best accounts for the polarity of methanol,CH3OH,and the bond polarities that make a major contribution to the overall molecular polarity? 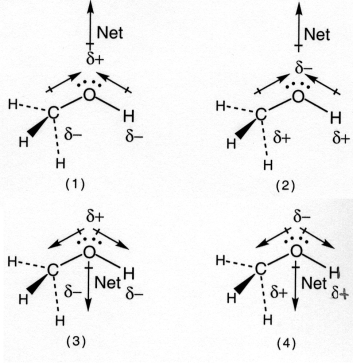
A) drawing (1)
B) drawing (2)
C) drawing (3)
D) drawing (4)
Correct Answer

verified
Correct Answer
verified
Showing 121 - 140 of 186
Related Exams