A) -440.8 kJ/mol
B) -416.9 kJ/mol
C) -1203.9 kJ/mol
D) -346.2 kJ/mol
E) -726.7 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
From the following thermochemical equations,calculate ΔrH° for the reaction: SO2(g) + NO2(g) → SO3(g) + NO(g) 2 SO2(g) + O2(g) → 2 SO3(g) ΔrH° = -197.8 kJ/mol 2 NO(g) + O2(g) → 2 NO2(g) ΔrH° = -114.14 kJ/mol
A) -83.66 kJ /mol
B) -311.9 kJ/mol
C) +155.9 kJ/mol
D) -155.9 kJ/mol
E) -41.83 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The specific heat capacity of liquid mercury is 0.14 J g-1k-1.How many joules of heat are needed to raise the temperature of 5.00 g of mercury from 15.0 °C to 36.5 °C?
A) 7.7 × 102 J
B) 15 J
C) 36 J
D) 0.0013 J
E) 1.7 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The complete combustion of propane,C3H8(g) ,is represented by the equation: C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(l) ΔrH° = -2220 kJ /mol How much heat is evolved in the complete combustion of 12.5 L C3H8(g) at 25 °C and 790 mmHg?
A) 8.27 × 104 kJ
B) 1.41 × 104 kJ
C) 653 kJ
D) 168 kJ
E) 1180 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate ΔfH° of octane,C8H18(l) ,given the enthalpy of combustion of octane to CO2(g) and H2O(l) ,-5471 kJ/mol,and the standard enthalpies of formation of CO2(g) and H2O(l) ,-393.5 kJ/mol and -285.8 kJ/mol,respectively.
A) -249.2 kJ/mol
B) +4792 kJ/mol
C) +249.2 kJ/mol
D) -4792 kJ/mol
Correct Answer

verified
Correct Answer
verified
True/False
A closed system can exchange matter but not energy with the surroundings.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Compute ΔrH° for the following reaction.The value of ΔfH° in kJ/mol is given below each species:
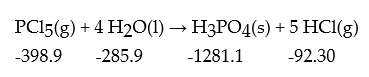
A) -688.6 kJ/mol
B) -200.1 kJ/mol
C) -74.9 kJ/mol
D) -1000.6 kJ/mol
E) -900.3 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the enthalpy change in the following equation: Cl2(g) + H2O(l) → 2 HCl(g) + 1/2 O2(g) ΔfH° H2O(l) = -285.8 kJ/mol ΔfH° HCl(g) = -92.31 kJ/mol
A) 193.5 kJ/mol
B) -470.4 kJ/mol
C) 470.4 kJ/mol
D) 101.2 kJ/mol
E) -101.2 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the INCORRECT statement.
A) Kinetic energy is the energy of motion.
B) Potential energy is energy in action.
C) Heat is energy transferred as a result of a temperature difference.
D) Pressure-volume work is calculated by w = −PΔV.
E) Heat is transferred from a warmer body to a colder one.
Correct Answer

verified
Correct Answer
verified
True/False
Kinetic energy is the energy of motion.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction H2(g) + 1/2 O2(g) → H2O(g) ΔrH° = -241.8 kJ/mol, What mass of H2(g) is required to liberate 1.00 × 103 kJ of heat?
A) 66.2 g
B) 4.17 g
C) 8.34 g
D) 2.05 g
E) 16.7 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 5.00 g sample of copper metal at 25.0 °C is heated by the addition of 96.0 J of energy.The final temperature of the copper is ________ °C.The specific heat capacity of copper is 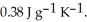
A) 32.3
B) 25.0
C) 25.5
D) 75.5
E) 50.5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Coal contains an impurity that reacts with oxygen during the combustion process,producing oxides that are major environmental pollutants.This impurity is:
A) acid rain
B) nitrogen
C) sulfur
D) carbon dioxide
E) peat
Correct Answer

verified
Correct Answer
verified
True/False
The standard enthalpy for diamond and graphite are the same value.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the work,w,gained or lost by the system when a gas expands from 15 L to 40 L against a constant external pressure of 1.5199 bar.
A) -6.1 kJ
B) -3.8 kJ
C) +3.8 kJ
D) +6.1 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
To have a standard enthalpy of formation referenced to 0 J/mol,the substance must: I.be a simple substance (chemical element) II.be in its most stable form III.be under 1 bar of pressure IV.have a concentration of 1.0000 M
A) I,III,IV
B) I,II,III
C) II,IV
D) I,III
E) II,III,IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The standard enthalpy of formation of NH4Cl(s) is -315.4 kJ/mol.The equation that describes this △fH° is:
A) NH4+(aq) + Cl-(aq) → NH4Cl(s)
B) 1/2 N2(g) + 2 H2(g) + 1/2 Cl2(g) → NH4Cl(s)
C) N2(g) + H2(g) + Cl2(g) → 2 NH4Cl(s)
D) 1/2 N2(g) + 1/2 Cl2(g) + 1/2 H2(g) → NH4Cl(s)
E) N2(g) + Cl2(g) + 4 H2(g) → 2 NH4Cl(s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction:
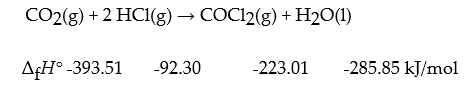 Compute ΔrH° for this reaction.
Compute ΔrH° for this reaction.
A) -994.67 kJ/mol
B) 23.05 kJ /mol
C) -23.05 kJ /mol
D) 69.25 kJ /mol
E) -69.25 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Oxygen gas at 34.5 °C expands from 34.5 L to 45.7 L against a constant pressure of 750 mmHg.What is the work done in joules by the system?
A) 1.12 × 103 J
B) -1.12 × 103 J
C) 9.09 × 104 J
D) -9.09 × 104 J
E) 4.55 × 103 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate ΔU for a system that loses 475 kJ of heat and does 155 kJ of expansion work on the surroundings.
A) -630 kJ
B) -320 kJ
C) +630 kJ
D) +320 kJ
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 125
Related Exams