A) 13.4
B) 33.6
C) 5.38
D) 0.0116
E) 3.75
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the correct statement about the equilibrium: N2(g) + 3 H2(g) ⇌ 2 NH3(g) ,Kp = 1 × 10-4
A) The rate constant for the forward reaction is greater than that of the reverse reaction.
B) Since the reaction has a high activation energy,a catalyst is not needed.
C) The equilibrium constant is given by Kp = [N2][H2]3/[NH3]2.
D) Conducting the reaction under high pressures will increase the yield of ammonia.
E) The Kp is independent of temperature.
Correct Answer

verified
Correct Answer
verified
True/False
Reaction quotient Q will always be equal to the reaction's equilibrium constant K.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following equilibrium. A(g) + 3 B(g) ⇌ 2 C(g) If the initial concentrations are [A] = 1.00 M,[B] = 3.00 M,and [C] = 0,at equilibrium it is found that [C] = 0.980 M.Calculate Kc for this reaction.
A) 0.526
B) 0.268
C) 1.26
D) 0.131
E) 1.901
Correct Answer

verified
Correct Answer
verified
True/False
In an equilibrium process,the concentrations of products and of reactants are equal.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following keep the equilibrium position unchanged?
A) temperature decrease
B) concentration change
C) temperature
D) pressure change
E) homogeneous catalyst
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the decomposition of SO3(g) ,Kc = [SO2]2[O2]/[SO3]2,at equilibrium,there are 0.090 mol SO2,0.110 mol O2,0.100 mol SO3 in a 25.0-L container.What is the value of Kc?
A) 3.6 × 10-3
B) 0.040
C) 2.23
D) 0.089
E) 7.89
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction: 3 Fe(s) + 4 H2O(g) ⇌ Fe3O4(s) + 4 H2(g) what is the effect of removing H2?
A) The reaction shifts to the right.
B) There is no change.
C) The reaction shifts to the left.
D) The Kp is decreased.
E) The Kp is doubled.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Dinitrogen tetroxide partially decomposes according to the following equilibrium:
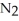
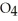 (g) ⇌
(g) ⇌ 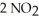 (g)
A 1.000 L flask is charged with 3.00 × 10-2 mol of
(g)
A 1.000 L flask is charged with 3.00 × 10-2 mol of 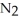
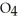 .At equilibrium,2.36 × 10-2 mol of
.At equilibrium,2.36 × 10-2 mol of 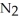
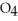 Remains.
Remains. 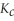 For this reaction is:
For this reaction is:
A) 0.723 mol L-1
B) 0.391 mol L-1
C) 0.212 mol L-1
D) 6.94 × ![]() mol L-1
mol L-1
E) 1.92 × 10-4 mol L-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction 2 A(g) ⇌ B(g) + C(g) ,Kc = 1.25 at 300 K.If a 1.00 L mixture contains 0.619 mol A,0.693 mol B,and 0.689 mol C at 300 K,will the mixture be in equilibrium? If not,in what direction will a net reaction occur?
A) Yes,reaction is at equilibrium.
B) No,net reaction proceeds to the left.
C) No,net reaction proceeds to the right.
D) No,but there is no net reaction.
E) Yes,net reaction proceeds to the right
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the exothermic reaction: 4 HCl(aq) + MnO2(s) ⇌ Cl2(g) + 2 H2O(l) + MnCl2(aq) The equilibrium is displaced to the left if:
A) catalyst is added
B) pressure is lowered
C) temperature is lowered
D) MnCl2(aq) is added
E) MnO2(s) is added
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction: 2 Cl2(g) + 2 H2O(g) ⇌ 4 HCl(g) + O2(g) ,Kp = 6.4 × 10-6 at 500 K.If a fixed volume is filled with initial concentrations of these gases at 227 °C such that [Cl2] = 0.5 M,[H2O] = 0.40 M,[HCl] = 0.5 M,and [O2] = 0.015 M,in which direction will the reaction proceed?
A) The reaction proceeds to the right.
B) The reaction proceeds to the left.
C) The reaction is already at equilibrium.
D) The reaction volume must be specified to answer this question.
E) The value of Kp at 25 °C must be specified to answer this question.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which factor influences the value of the equilibrium constant for a reversible reaction?
A) addition of a catalyst
B) raising the temperature
C) removing product
D) removing reactant
E) increase in mixing rate
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the equilibrium system: N2O4(g) ⇌ 2 NO2(g) for which Kp = 0.1134 at 25 °C and ΔrH° = 58.03 kJ/mol.Assuming that the total pressure inside the container is 1.00 atm at equilibrium and that initially only N2O4 was present inside the container,compute 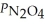 At equilibrium.
At equilibrium.
A) 0.398 atm
B) 0.113 atm
C) 0.602 atm
D) 0.285 atm
E) 0.715 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction: CH4(g) + 2 H2O(g) ⇌ CO2(g) + 4 H2(g) ,ΔrH° = +190 kJ,add N2(g) at constant volume and:
A) the reaction reacts to the right
B) the reaction reacts to the left
C) the ΔH° increases
D) the temperature increases
E) there is no change
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction: CH4(g) + 2 H2O(g) ⇌ CO2(g) + 4 H2(g) ,ΔrH° = +190 kJ,when catalyst is added:
A) the reaction shifts to the right
B) the reaction shifts to the left
C) the ΔH° increases
D) the temperature increases
E) there is no change,catalyst changes reaction rate only
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At a certain temperature,Kc equals 1.40 × 102 M-1 for the reaction: 2 CO(g) + O2(g) ⇌ 2 CO2(g) If a 3.00 L flask contains 0.400 mol of CO2 and 0.100 mol of O2 at equilibrium,how many moles of CO are also present in the flask?
A) 0.555 mol
B) 0.185 mol
C) 0.107 mol
D) 0.0114 mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An equilibrium mixture of CO,O2,and CO2 at a certain temperature contains 0.0010 mol L-1 CO2 and 0.0015 mol L-1 O2.At this temperature,Kc equals 1.4 × 102 L for the reaction: 2 CO(g) + O2(g) ⇌ 2 CO2(g) What is the equilibrium concentration of CO?
A) 4.8 × 10-6 mol L-1
B) 8.5 × 10-3 mol L-1
C) 9.3 × 10-2 mol L-1
D) 3.1 × 10-1 mol L-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction. C(s) + H2O(g) ⇌ CO(g) + H2(g) At equilibrium at a certain temperature,[H2O] = 0.12 M,and [CO] = [H2] = 1.2 M.If suddenly these concentrations are increased by 0.50 M,which of the following is true?
A) more products are formed
B) Kc = 4.66
C) more H2O(g) will be formed
D) Since Kc does not change,nothing happens.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction: 3 Fe(s) + 4 H2O(g) ⇌ Fe3O4(s) + 4 H2(g) what is the effect on equilibrium of increasing temperature of an exothermic reaction?
A) The reaction shifts to the right.
B) There is no change.
C) The reaction shifts to the left.
D) The Kp is decreased.
E) The Kp is doubled.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 118
Related Exams