A) ![]() O + S
O + S ![]() →
→ ![]() (aq)
(aq)
B) 2 ![]() O + 2 S
O + 2 S ![]() → 2
→ 2 ![]()
![]() (aq) +
(aq) + ![]() (g)
(g)
C) ![]() O + S
O + S ![]() → S
→ S ![]() (g) +
(g) + ![]() (g)
(g)
D) ![]() O + S
O + S ![]() → No Reaction
→ No Reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the source of acid rain?
A) Acid rain is from dissolved carbon dioxide.
B) All rain is acid rain because rain has a pH is less than 7.
C) Rain is normally basic, but depending on the weather it can get slightly acidic.
D) All rain is acid rain because rain has a hydronium ion concentration greater than 10-7 M.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A weak acid is added to a concentrated solution of hydrochloric acid. Does the solution become more or less acidic?
A) More acidic, since there are more hydronium ions being added to the solution.
B) Less acidic, since the solution becomes more dilute with a less concentrated solution of hydronium ions being added to the solution.
C) No change in acidity, since the concentration of the hydrochloric acid is too high to be changed by the weak solution.
D) Less acidic since the concentration of hydroxide ions will increase.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about strong and weak bases is not true?
A) A weak base does not completely dissociate in water.
B) A weak base will not react with a strong acid.
C) A strong base can be extremely corrosive.
D) A strong base will readily accept protons from even weak acids.
E) All of the above are untrue.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is phosphoric acid, 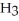
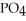 , a stronger acid than disodium hydrogen phosphate,
, a stronger acid than disodium hydrogen phosphate, 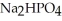 ?
? 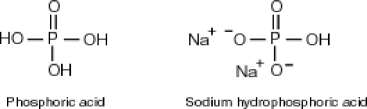
A) Phosphoric acid has three acidic hydrogens which makes it three times as acidic.
B) Some of the released sodium ions in ![]() HP
HP ![]() form NaOH (a base) , which decreases the acidity of the
form NaOH (a base) , which decreases the acidity of the ![]() HP
HP ![]() .
.
C) Phosphoric acid dissociates 100% in water whereas ![]() HP
HP ![]() only dissociates about 50%.
only dissociates about 50%.
D) None of the above accurately describes why phosphoric acid is a stronger acid than disodium hydrogen phosphate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following acid-base reaction, identify what is formed in the space marked. HF + KOH ⇌ ???? + H2O
A) KF
B) H3OF
C) KOH2
D) KOH2F
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements describes a neutral solution?
A) [H3O+] > [OH-]
B) [H3O+] < [OH-]
C) [H3O+] × [OH-] ≠ 1 × 10-14
D) [H3O+]/[OH-] = 1 × 10-14
E) [H3O+]/[OH-] = 1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the following reaction, which molecule is acting as an acid? H3O+ + HSO4- → H2O + H2SO4
A) H2SO4
B) H2O
C) H3O+
D) HSO4-
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why do we use the pH scale to indicate the acidity of a solution rather than simply stating the concentration of hydronium ions?
A) It includes the concentration of hydronium and hydroxide ions.
B) It is used because the general public understands it.
C) It is more accurate to use the pH scale.
D) It is more convenient, since the concentration of hydronium ions is so small.
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
The amphoteric reaction between two water molecules is endothermic, which means that this reaction requires the input of energy in order to proceed: Energy + 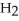 O +
O + 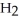 O →
O → 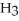
 + O
+ O 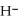 The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of
The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of 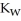 be expected to increase, decrease, or stay the same with increasing temperature?
be expected to increase, decrease, or stay the same with increasing temperature?
A) The value of ![]() will stay the same, since it is a constant.
will stay the same, since it is a constant.
B) The value of ![]() will decrease, since there are more hydronium ions than hydroxide ions.
will decrease, since there are more hydronium ions than hydroxide ions.
C) The value of ![]() will increase since the rise in temperature allows for a higher concentration of both ions.
will increase since the rise in temperature allows for a higher concentration of both ions.
D) The value of ![]() will decrease since there are more hydroxide ions than hydronium ions.
will decrease since there are more hydroxide ions than hydronium ions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following buffer system, what happens to the concentration of the highlighted molecule if you add acid in the form of H3O+? HF + NaF + H2O ⇌ F- + H3O+ + Na+
A) increases
B) decreases
C) stays about the same
D) Buffers do not change.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would be the concentration of hydronium ions in a solution that had a pH = -3? Why would such a solution be impossible to prepare?
A) Concentration of ![]()
![]() = 3 M. It is impossible because it is hard to measure such a small amount of hydronium ions accurately.
= 3 M. It is impossible because it is hard to measure such a small amount of hydronium ions accurately.
B) Concentration of ![]()
![]() = 30 M. It is impossible because pH can not be negative.
= 30 M. It is impossible because pH can not be negative.
C) Concentration of ![]()
![]() =
= ![]() M. It is impossible because any container used to hold the acid would immediately corrode.
M. It is impossible because any container used to hold the acid would immediately corrode.
D) Concentration of ![]()
![]() =
= ![]() M. It is impossible because only so much acid can dissolve in water before the solution becomes saturated.
M. It is impossible because only so much acid can dissolve in water before the solution becomes saturated.
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Cutting back on the pollutants that cause acid rain is one solution to the problem of acidified lakes. Suggest another.
A) stop using NaCl to salt the roads in the winter
B) add a neutralizing substance such as limestone
C) add ammonium ions to the lakes
D) add chlorine and filter the water to remove any acidity in the lake
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following acid-base reaction, identify what is formed in the space marked. H2SO4 + KOH ⇌ KHSO4 + ???
A) K2SO4
B) H3OSO4
C) H2O
D) KOH2SO4
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sometimes an individual going through a traumatic experience cannot stop hyperventilating. In such a circumstance, it is recommended that the individual breath into a paper bag or cupped hands as a useful way to avoid an increase in blood pH, which can cause the person to pass out. Explain how this works.
A) Carbon dioxide initially exhaled is reinhaled to help maintain adequate levels of carbonic acid in the blood.
B) It helps to slow their breathing down to prevent excess oxygen from entering the blood.
C) The paper absorbs C ![]() to prevent the accumulation of excess C
to prevent the accumulation of excess C ![]() in the blood.
in the blood.
D) The paper bag helps to retain the water vapor leaving the mouth to prevent an increase in the concentration of lactic acid in the body.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements describes an acidic solution?
A) [H3O+] > [OH-]
B) [H3O+] < [OH-]
C) [H3O+] × [OH-] ≠ 1 × 10-14
D) [H3O+]/[OH-] = 1 × 10-14
E) [H3O+]/[OH-] = 1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would be the best explanation for the fact that most natural water has a pH of about 5.6?
A) Carbon dioxide reacts with water to form an acid.
B) Air contains acid rain particles.
C) Minerals that are dissolved in water are often acidic.
D) Many salts that dissolve in natural waters make the water basic.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the following reaction, which molecule is acting as a base? H2O + NH3 → OH- + NH4+
A) H2O
B) NH3
C) OH-
D) NH4+
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Would it be easier or harder for a water molecule to donate a hydrogen ion if its polar H-O bond were even more polar? Why?
A) Easier. As polarity increases the bond classification tends towards ionic. An ionic bond would mean a greater separation of positive and negative charge making it easier for the water molecule to donate a hydrogen ion.
B) Harder. Increasing the polarity makes the bond stronger. The stronger the bond the more difficult it would be for the water molecule to donate a hydrogen ion.
C) Neither. Increasing the polarity of the O-H bond would not affect the hydrogen bonding between the water molecules and therefore have no effect on the water molecule's ability to donate a hydrogen ion.
D) Both. The ability of the water molecule to donate a hydrogen ion is dependent upon the environment you place it in. If the surrounding molecules are more covalent, donating the electron would be harder. However, if water found itself in a highly polar environment, increasing the O-H polarity would make it easier.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
As the pH increases, the hydroxide ion concentration ________.
A) goes down
B) gets larger
C) starts to affect the H+ concentration
D) starts to decrease because it is reacting with the excess hydronium ions
E) stays constant
Correct Answer

verified
B
Correct Answer
verified
Showing 1 - 20 of 135
Related Exams