A) 27
B) 7.35
C) 0.136
D) 2.92 × 103
E) 3.43 × 10-4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Acetic acid is a weak acid that dissociates into the acetate ion and a proton in aqueous solution: HC2H3O2 (aq) ![Acetic acid is a weak acid that dissociates into the acetate ion and a proton in aqueous solution: HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub> (aq) C<sub>2</sub>H<sub>3</sub>O<sub>2</sub><sup>-</sup> (aq) + H<sup>+</sup> (aq) At equilibrium at 25 °C a 0.100 M solution of acetic acid has the following concentrations: [HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>] = 0.0990 M, [C<sub>2</sub>H<sub>3</sub>O<sub>2</sub><sup>-</sup>] = 1.33 × 10<sup>-3</sup><sup> </sup>M and [H+] = 1.33 × 10<sup>-3</sup><sup> </sup>M. The equilibrium constant, K<sub>eq</sub>, for the ionization of acetic acid at 25 °C is ________. A) 5.71 × 10<sup>4</sup> B) 0.100 C) 1.75 × 10<sup>-7</sup> D) 1.79 × 10<sup>-5</sup> E) 5.71 × 10<sup>6</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f6a_9fa5_a2ab_afb72bbd682d_TB2701_11.jpg) C2H3O2- (aq) + H+ (aq)
At equilibrium at 25 °C a 0.100 M solution of acetic acid has the following concentrations:
[HC2H3O2] = 0.0990 M, [C2H3O2-] = 1.33 × 10-3 M and [H+] = 1.33 × 10-3 M. The equilibrium constant, Keq, for the ionization of acetic acid at 25 °C is ________.
C2H3O2- (aq) + H+ (aq)
At equilibrium at 25 °C a 0.100 M solution of acetic acid has the following concentrations:
[HC2H3O2] = 0.0990 M, [C2H3O2-] = 1.33 × 10-3 M and [H+] = 1.33 × 10-3 M. The equilibrium constant, Keq, for the ionization of acetic acid at 25 °C is ________.
A) 5.71 × 104
B) 0.100
C) 1.75 × 10-7
D) 1.79 × 10-5
E) 5.71 × 106
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The value of Keq for the following reaction is 0.25: SO2 (g) + NO2 (g)  SO3 (g) + NO (g)
The value of Keq at the same temperature for the reaction below is ________.
3SO2 (g) + 3NO2 (g)
SO3 (g) + NO (g)
The value of Keq at the same temperature for the reaction below is ________.
3SO2 (g) + 3NO2 (g)  3SO3 (g) + 3NO (g)
3SO3 (g) + 3NO (g)
A) 1.6 × 10-2
B) 7.5 × 10-1
C) 8.3 × 10-2
D) 6.4 × 101
E) 0.25
Correct Answer

verified
Correct Answer
verified
Short Answer
For an exothermic reaction, increasing the reaction temperature results in a(n)________ in K.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following expressions is the correct equilibrium-constant expression for the reaction below? (NH4) 2Se (s) ![Which of the following expressions is the correct equilibrium-constant expression for the reaction below? (NH<sub>4</sub>) <sub>2</sub>Se (s) 2NH<sub>3</sub> (g) + H<sub>2</sub>Se (g) A) [NH<sub>3</sub>][H<sub>2</sub>Se] / [(NH<sub>4</sub>) <sub>2</sub>Se] B) [(NH<sub>4</sub>) <sub>2</sub>Se] / [NH<sub>3</sub>]<sup>2</sup>[H<sub>2</sub>Se] C) 1 / [(NH<sub>4</sub>) <sub>2</sub>Se] D) [NH<sub>3</sub>]<sup>2</sup>[H<sub>2</sub>Se] E) [NH<sub>3</sub>]<sup>2</sup>[H<sub>2</sub>Se] / [(NH<sub>4</sub>) <sub>2</sub>Se]](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f67_6c17_a2ab_ed8418460b40_TB2701_11.jpg) 2NH3 (g) + H2Se (g)
2NH3 (g) + H2Se (g)
A) [NH3][H2Se] / [(NH4) 2Se]
B) [(NH4) 2Se] / [NH3]2[H2Se]
C) 1 / [(NH4) 2Se]
D) [NH3]2[H2Se]
E) [NH3]2[H2Se] / [(NH4) 2Se]
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following chemical reaction: CO (g) + 2H2(g)  CH3OH(g)
At equilibrium in a particular experiment, the concentrations of CO and H2 were 0.15 M and 0.36, M respectively. What is the equilibrium concentration of CH3OH? The value of Keq for this reaction is 14.5 at the temperature of the experiment.
CH3OH(g)
At equilibrium in a particular experiment, the concentrations of CO and H2 were 0.15 M and 0.36, M respectively. What is the equilibrium concentration of CH3OH? The value of Keq for this reaction is 14.5 at the temperature of the experiment.
A) 14.5
B) 7.61 × 10-3
C) 2.82 × 10-1
D) 3.72 × 10-3
E) 1.34 × 10-3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following equilibria, only ________ will shift to the right in response to a decrease in volume.
A) H2 (g) + Cl2 (g) ![]() 2 HCl (g)
2 HCl (g)
B) 2 SO3 (g) ![]() 2 SO2 (g) + O2 (g)
2 SO2 (g) + O2 (g)
C) N2 (g) + 3H2 (g) ![]() 2NH3 (g)
2NH3 (g)
D) 2 Fe2O3 (s) ![]() 4 Fe (s) + 3O2 (g)
4 Fe (s) + 3O2 (g)
E) 2HI (g) ![]() H2 (g) + I2 (g)
H2 (g) + I2 (g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Keq for the equilibrium below is 0.112 at 700.0 °C. SO2 (g) +  O2 (g)
O2 (g)  SO3 (g)
What is the value of Keq at this temperature for the following reaction?
2SO2 (g) + O2 (g)
SO3 (g)
What is the value of Keq at this temperature for the following reaction?
2SO2 (g) + O2 (g)  2SO3 (g)
2SO3 (g)
A) 1.25 × 10-2
B) 2.24 × 10-1
C) 7.97 × 101
D) 4.46
E) 0.112
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction vessel is charged with hydrogen iodide, which partially decomposes to molecular hydrogen and iodine: 2HI (g)  H2(g) + I2(g)
When the system comes to equilibrium at 425 °C, PHI = 0.708 atm, and PH2 = PI2 = 0.0960 atm. The value of Kp at this temperature is ________.
H2(g) + I2(g)
When the system comes to equilibrium at 425 °C, PHI = 0.708 atm, and PH2 = PI2 = 0.0960 atm. The value of Kp at this temperature is ________.
A) 6.80 × 10-2
B) 1.30 × 10-2
C) 54.3
D) 1.84 × 10-2
E) Kp cannot be calculated for this gas reaction when the volume of the reaction vessel is not given.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction at equilibrium. 2CO2 (g)  2CO (g) + O2 (g) ΔH° = -514 kJ
Le Châtelier's principle predicts that the equilibrium partial pressure of CO (g) can be maximized by carrying out the reaction ________.
2CO (g) + O2 (g) ΔH° = -514 kJ
Le Châtelier's principle predicts that the equilibrium partial pressure of CO (g) can be maximized by carrying out the reaction ________.
A) at high temperature and high pressure
B) at high temperature and low pressure
C) at low temperature and low pressure
D) at low temperature and high pressure
E) in the presence of solid carbon
Correct Answer

verified
Correct Answer
verified
Short Answer
Exactly 3.5 moles if N2O4 is placed in an empty 2.0-L container and allowed to reach equilibrium described by the equation N2O4 (g)⇌ 2NO2 (g) If at equilibrium the N2O4 is 25% dissociated, what is the value of the equilibrium constant for the reaction?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitrosyl bromide decomposes according to the following equation. 2NOBr (g)  2NO (g) + Br2 (g)
A sample of NOBr (0.64 mol) was placed in a 1.00-L flask containing no NO or Br2. At equilibrium the flask contained 0.16 mol of NOBr. How many moles of NO and Br2, respectively, are in the flask at equilibrium?
2NO (g) + Br2 (g)
A sample of NOBr (0.64 mol) was placed in a 1.00-L flask containing no NO or Br2. At equilibrium the flask contained 0.16 mol of NOBr. How many moles of NO and Br2, respectively, are in the flask at equilibrium?
A) 0.48, 0.24
B) 0.48, 0.48
C) 0.16, 0.08
D) 0.16, 0.16
E) 0.24, 0.42
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction at equilibrium: 2CO2 (g)  2CO (g) + O2 (g) ΔH° = -514 kJ
Le Châtelier's principle predicts that an increase in temperature will ________.
2CO (g) + O2 (g) ΔH° = -514 kJ
Le Châtelier's principle predicts that an increase in temperature will ________.
A) increase the partial pressure of O2 (g)
B) decrease the partial pressure of CO2 (g)
C) decrease the value of the equilibrium constant
D) increase the value of the equilibrium constant
E) increase the partial pressure of CO
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What role did Karl Bosch play in development of the Haber-Bosch process?
A) He discovered the reaction conditions necessary for formation of ammonia.
B) He originally isolated ammonia from camel dung and found a method for purifying it.
C) Haber was working in his lab with his instructor at the time he worked out the process.
D) He developed the equipment necessary for industrial production of ammonia.
E) He was the German industrialist who financed the research done by Haber.
Correct Answer

verified
Correct Answer
verified
True/False
At constant temperature, reducing the volume of a gaseous equilibrium mixture causes the reaction to shift in the direction that increases the number of moles of gas in the system.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The value of Keq for the equilibrium H2 (g) + I2 (g)  2HI (g)
Is 794 at 25 °C. What is the value of Keq for the equilibrium below?
2HI (g)
Is 794 at 25 °C. What is the value of Keq for the equilibrium below? 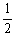 H2 (g) +
H2 (g) + 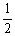 I2 (g)
I2 (g)  HI (g)
HI (g)
A) 397
B) 0.035
C) 28
D) 1588
E) 0.0013
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The value of Keq for the equilibrium H2 (g) + I2 (g)  2HI (g)
Is 794 at 25 °C. At this temperature, what is the value of Keq for the equilibrium below?
HI (g)
2HI (g)
Is 794 at 25 °C. At this temperature, what is the value of Keq for the equilibrium below?
HI (g) 
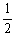 H2 (g) +
H2 (g) + 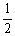 I2 (g)
I2 (g)
A) 1588
B) 28
C) 397
D) 0.035
E) 0.0013
Correct Answer

verified
Correct Answer
verified
Short Answer
If the value for the equilibrium constant is much greater than 1, then the equilibrium mixture contains mostly ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The expression for Kp for the reaction below is ________. 4CuO (s) + CH4 (g)  CO2 (g) + 4Cu (s) + 2H2O (g)
CO2 (g) + 4Cu (s) + 2H2O (g)
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following expressions is the correct equilibrium-constant expression for the equilibrium between dinitrogen tetroxide and nitrogen dioxide? 5N2O4(g) ![Which of the following expressions is the correct equilibrium-constant expression for the equilibrium between dinitrogen tetroxide and nitrogen dioxide? 5N<sub>2</sub>O4(g) 10NO<sub>2</sub> (g) A) [NO<sub>2</sub>]<sup>10</sup>/[N<sub>2</sub>O<sub>4</sub>]<sup>5</sup> B) [N<sub>2</sub>O<sub>4</sub>]<sup>10</sup>/[NO<sub>2</sub>]<sup>5</sup> C) [NO<sub>2</sub>]<sup>5</sup>/[N<sub>2</sub>O<sub>4</sub>]<sup>10</sup> D) [NO<sub>2</sub>]<sup>5</sup>/[N<sub>2</sub>O<sub>4</sub>]<sup>5</sup> E) [N<sub>2</sub>O<sub>4</sub>]<sup>5</sup>/[NO<sub>2</sub>]<sup>5</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f6b_14dc_a2ab_f36dc15fb491_TB2701_11.jpg) 10NO2 (g)
10NO2 (g)
A) [NO2]10/[N2O4]5
B) [N2O4]10/[NO2]5
C) [NO2]5/[N2O4]10
D) [NO2]5/[N2O4]5
E) [N2O4]5/[NO2]5
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 97
Related Exams