Correct Answer

verified
Correct Answer
verified
Multiple Choice
The relative initial rates of the reaction A2 + B2 → products in vessels (a) -(d) are 1:1:4:4.Unshaded spheres represent A2 molecules,and shaded spheres represent B2 molecules present at the beginning of the reaction. ![The relative initial rates of the reaction A<sub>2</sub> + B<sub>2</sub> → products in vessels (a) -(d) are 1:1:4:4.Unshaded spheres represent A<sub>2</sub> molecules,and shaded spheres represent B<sub>2</sub> molecules present at the beginning of the reaction. -What is the rate law for this reaction? A) Rate = k[A<sub>2</sub>]<sup>2</sup> B) Rate = k[B<sub>2</sub>]<sup>2</sup> C) Rate = k[A<sub>2</sub>][B<sub>2</sub>] D) Rate = k[A<sub>2</sub>]<sup>2</sup>[B<sub>2</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d158_627e_a2f7_038778bc1d6e_TB4940_00_TB4940_00_TB4940_00_TB4940_00.jpg) -What is the rate law for this reaction?
-What is the rate law for this reaction?
A) Rate = k[A2]2
B) Rate = k[B2]2
C) Rate = k[A2][B2]
D) Rate = k[A2]2[B2]2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hydrolysis of tert-butyl chloride is given in the reaction below: (CH3) 3CCl(aq) + H2O(l) → (CH3) 3COH(aq) + H+(aq) + Cl-(aq) If the rate law is: Rate = k[(CH3) 3CCl],what is the order of the reaction with respect to water?
A) zero
B) first
C) second
D) third
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate constant,k,for a first-order reaction is equal to 4.2 × 10-4 s-1.What is the half-life for the reaction?
A) 2.9 × 10-4 s
B) 1.2 × 103 s
C) 1.7 × 103 s
D) 2.4 × 103 s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The second-order reaction,2 Mn(CO) 5 → Mn2(CO) 10 has a rate constant equal to 3.0 × 109 M-1s-1 at 25°C.If the initial concentration of Mn(CO) 5 is 1.0 × 10-5 M,how long will it take for 90.% of the reactant to disappear?
A) 3.3 × 10-16 s
B) 3.7 × 10-15 s
C) 3.0 × 10-4 s
D) 3.0 × 103 s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following set of data was obtained by the method of initial rates for the reaction: BrO3-(aq) + 5 Br-(aq) + 6 H+(aq) → 3 Br2(aq) + 3 H2O(l) .
Calculate the initial rate when BrO3- is 0.30 M,Br- is 0.050 M,and H+ is 0.15 M. 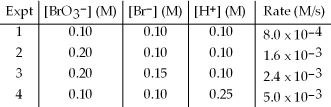
A) 6.1 × 10-5 M/s
B) 2.7 × 10-3 M/s
C) 5.3 × 10-2 M/s
D) 8.4 × 10-2 M/s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the rate law for the elementary reaction shown below? 2 HI → H2 + I2
A) Rate = k[HI]
B) Rate = k[HI]2
C) Rate = k[H2][I2]
D) Rate = k[H2][I2]/[HI]2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The aquation of tris(1,10-phenanthroline) iron(II) in acid solution takes place according to the equation: Fe(phen) 32+ + 3 H3O+ + 3 H2O → Fe(H2O) 62+ + 3 phenH+ If the activation energy,Ea,is 126 kJ/mol and the rate constant at 30°C is 9.8 × 10-3 min-1,what is the frequency factor,A?
A) 2 × 10-24 min-1
B) 2 × 10-20 min-1
C) 5 × 1019 min-1
D) 5 × 1023 min-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the temperature of a gas whose activation energy is 55 kJ/mol is increased from 300 K to 320 K,the fraction of collisions with sufficient energy to react
A) decreases by a factor of 2.
B) decreases by a factor of 4.
C) increases by a factor of 2.
D) increases by a factor of 4.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitrogen dioxide decomposes at 300°C via a second-order process to produce nitrogen monoxide and oxygen according to the following chemical equation. 2 NO2(g) → 2 NO(g) + O2(g) . A sample of NO2(g) is initially placed in a 2.50-L reaction vessel at 300°C.If the half-life and the rate constant at 300°C are 11 seconds and 0.54 M-1 s-1,respectively,how many moles of NO2 were in the original sample?
A) 0.17 mol
B) 0.42 mol
C) 5.9 mol
D) 15 mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to kinetic molecular theory,which of the following will decrease the rate of reaction?
A) increase the temperature
B) increase the concentration
C) increase the size of the molecule
D) increase the size of the reaction vessel
Correct Answer

verified
Correct Answer
verified
Short Answer
The reaction shown below has the rate law: Rate = k[BrO3-][Br-][H+]2.The order of reaction with respect to H+ is ________ and the overall order is ________. BrO3-(aq)+ 5 Br-(aq)+ 6 H+(aq)→ 3 Br2(aq)+ 3 H2O(l)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The first-order reaction,SO2Cl2 → SO2 + Cl2,has a rate constant equal to 2.20 × 10-5 s-1 at 593 K.What percentage of the initial amount of SO2Cl2 will remain after 2.00 hours?
A) 1.00%
B) 14.7%
C) 17.1%
D) 85.4%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Molecular hydrogen can be made from methane gas by the reaction below.How is the rate of disappearance of CH4 related to the rate of appearance of H2? - 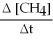 = ?
CH4 (g) + H2O (l) → CO (g) + 3H2 (g)
= ?
CH4 (g) + H2O (l) → CO (g) + 3H2 (g)
A) + ![]()
B) + ![]()
C) + 3 ![]()
D) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following does not affect the rate of a bimolecular reaction?
A) concentrations of reactants
B) presence of a catalyst
C) temperature
D) All of these affect the rate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction between chlorine and nitric oxide to form nitrosyl chloride is shown below.If the reaction rate doubles when the concentration of Cl2 is doubled and the rate quadruples when the concentration of NO is doubled,by what factor will the rate increase if both concentrations,NO and Cl2,are doubled? Cl2(g) + 2 NO(g) → 2 NOCl(g)
A) 2
B) 4
C) 8
D) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Biological reactions are catalyzed by
A) sugar.
B) enzymes.
C) fats.
D) water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
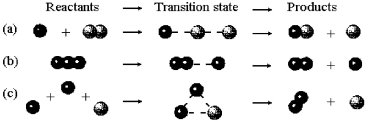 -Which of the elementary reactions shown above has a molecularity of three?
-Which of the elementary reactions shown above has a molecularity of three?
A) elementary reaction (a)
B) elementary reaction (b)
C) elementary reaction (c)
D) elementary reactions (a) , (b) ,and (c)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction is first order,C2H6 → 2 CH3.If the rate constant is equal to 5.5 × 10-4 s-1 at 1000 K,how long will it take for 0.35 mol of C2H6 in a 1.00 L container to decrease to 0.25 mol in the same container?
A) 3.0 min
B) 10.min
C) 42 min
D) 48 min
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The half life of the reaction shown below is found not to depend on the concentration of H2O2(aq) . 2 H2O2(aq) → 2 H2O(l) + O2(g) What is the order of this reaction?
A) zeroth
B) first
C) second
D) third
Correct Answer

verified
Correct Answer
verified
Showing 141 - 160 of 190
Related Exams