A) sp
B) sp3d2
C) sp3d
D) sp3
E) sp2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) ,molecular geometry (mg) ,and polarity of N2O (N central) .
A) eg = linear,mg = linear,nonpolar
B) eg = tetrahedral,mg = linear,nonpolar
C) eg = tetrahedral,mg = bent,polar
D) eg = linear,mg = linear,polar
E) eg = trigonal planar,mg = bent,polar
Correct Answer

verified
Correct Answer
verified
Essay
According to molecular orbital theory,what is an antibonding orbital?
Correct Answer

verified
An antibonding orbital is formed when tw...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
A molecule containing a central atom with sp2 hybridization has a ________ electron geometry.
A) linear
B) trigonal bipyramidal
C) trigonal planar
D) tetrahedral
E) bent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the molecule below.Determine the hybridization at each of the two labelled carbons. 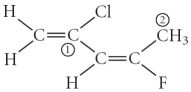
A) C1 = sp3,C2 = sp3d
B) C1 = sp,C2 = sp2
C) C1 = sp2,C2 = sp3d
D) C1 = sp3d,C2 = sp3d2
E) C1 = sp2,C2 = sp3
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
A molecule or ion with four electrons in bonding orbitals and two electrons in an antibonding orbital has a bond order of:
A) 1
B) 1.5
C) 2
D) 2.5
E) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the molecular geometry for the molecule PF3.
A) Trigonal pyramidal
B) Trigonal planar
C) Tetrahedral
D) T-shaped
E) Bent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) ,molecular geometry (mg) ,and polarity of XeO3.
A) eg = trigonal planar,mg = trigonal planar,nonpolar
B) eg = tetrahedral,mg = trigonal pyramidal,polar
C) eg = trigonal planar,mg = trigonal pyramidal,polar
D) eg = trigonal bipyramidal,mg = trigonal planar,nonpolar
E) eg = octahedral,mg = tetrahedral,nonpolar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules are polar? XeO2 SiCl2Br2 C2Br2 SeCl6
A) 1
B) 4
C) 2
D) 3
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the molecular geometry for the molecule BrF3.
A) Square pyramidal
B) Seesaw
C) T-shaped
D) Square planar
E) Trigonal planar
Correct Answer

verified
Correct Answer
verified
Essay
Use molecular orbital theory to determine whether He22+ or He2+ is more stable.
Correct Answer

verified
The He22+ ion is more stable sinc...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Give the electron geometry (eg) ,molecular geometry (mg) ,and hybridization for CH3-.
A) eg = tetrahedral; mg = trigonal pyramidal; sp3
B) eg = tetrahedral; mg = tetrahedral; sp3
C) eg = trigonal pyramidal; mg = trigonal pyramidal; sp3
D) eg = trigonal planar; mg = trigonal planar; sp2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
List the number of sigma bonds and pi bonds in a triple bond.
A) 1 sigma,1 pi
B) 2 sigma,1 pi
C) 3 sigma,0 pi
D) 1 sigma,2 pi
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules are polar? XeCl2 COF2 PCl4F SF6
A) 0
B) 3
C) 1
D) 2
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule or ion with five electrons in bonding orbitals and two electrons in an antibonding orbital has a bond order of:
A) 0.5
B) 1
C) 1.5
D) 2
E) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following in order of decreasing X-A-X bond angle,where A represents the central atom and X represents the outer atoms in each molecule. N2O NCl3 NO2-
A) NCl3 > NO2- > N2O
B) NO2- > N2O > NCl3
C) N2O > NO2- > NCl3
D) NCl3 > N2O > NO2-
E) N2O > NCl3 > NO2-
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for H3O+.What is the hybridization on the O atom?
A) sp
B) sp3
C) sp2
D) sp3d
E) sp3d2
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) ,molecular geometry (mg) ,and polarity of SF6.
A) eg = trigonal bipyramidal,mg = trigonal bipyramidal,nonpolar
B) eg = tetrahedral,mg = tetrahedral,polar
C) eg = trigonal bipyramidal,mg = seesaw,polar
D) eg = octahedral,mg = trigonal bipyramidal,nonpolar
E) eg = octahedral,mg = octahedral,nonpolar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the number of electron groups around a molecule with a trigonal bipyramidal shape.
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the approximate bond angle for a molecule with an octahedral shape.
A) 109.5°
B) 180°
C) 120°
D) 105°
E) 90°
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 158
Related Exams