A) Br < Se < Te < Sb
B) Se < Br < Sb < Te
C) Se < Br < Te < As
D) Sb < Te < Se < Br
E) Te < Sb < Se < Br
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following sets of quantum numbers is allowed?
A) n = 2, ![]() = 1,
= 1, ![]() = +1/2,ms = -1/2
= +1/2,ms = -1/2
B) n = 3, ![]() = 2,
= 2, ![]() = +1,ms = +1
= +1,ms = +1
C) n = 4, ![]() = 1,
= 1, ![]() = 0,ms = -1/2
= 0,ms = -1/2
D) n = 4, ![]() = 3,
= 3, ![]() = -1,ms = 0
= -1,ms = 0
E) n = 5, ![]() = 2,
= 2, ![]() = +2,ms = -1
= +2,ms = -1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the correct valence shell orbital box notation for the ground state electron configuration of Co? 3d 4s
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hund's rule states that the most stable arrangement of electrons (for a ground state electron configuration)
A) has a filled valence shell of electrons.
B) has three electrons per orbital,each with identical spins.
C) has ![]() values greater than or equal to +1.
values greater than or equal to +1.
D) has the maximum number of unpaired electrons,all with the same spin.
E) has two electrons per orbital,each with opposing spins.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom of which of the following elements has the smallest ionization energy?
A) Cl
B) P
C) Si
D) Na
E) S
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons can be described by the quantum numbers n= 3 and l= 1?
A) 10
B) 2
C) 14
D) 18
E) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which 1+ ion has the ground state electron configuration [Kr]4d10?
A) Ru
B) Au
C) Ag
D) In
E) Cd
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is a possible set of quantum numbers for an unpaired electron in the orbital box diagram below? 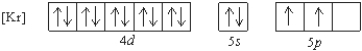
A) n = 1, ![]() = 1,
= 1, ![]() = -1,ms = +1/2
= -1,ms = +1/2
B) n = 4, ![]() = 2,
= 2, ![]() = -1,ms = -1/2
= -1,ms = -1/2
C) n = 5, ![]() = 2,
= 2, ![]() = -2,ms = +1/2
= -2,ms = +1/2
D) n = 5, ![]() = 0,
= 0, ![]() = 0,ms = -1/2
= 0,ms = -1/2
E) n = 5, ![]() = 1,
= 1, ![]() = -1,ms = +1/2
= -1,ms = +1/2
Correct Answer

verified
Correct Answer
verified
Short Answer
As one moves horizontally from left to right across a period,the effective ________ charge increases,resulting in decreasing atomic radii.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many unpaired electrons are found in the ground state electron configuration of mercury (Hg) ?
A) 0
B) 1
C) 2
D) 3
E) 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electron configuration of sulfur (S) ?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the charge formed by alkaline earth metals when they react with nonmetals?
A) +1
B) (-1)
C) +2
D) (-2)
E) +3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The change in energy for which of the following processes corresponds to the first ionization energy of calcium?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element has the following ground state electron configuration? 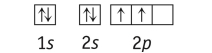
A) B
B) Li
C) Si
D) Be
E) C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An element that has the same ground state valence-shell electron configuration as germanium is
A) tin.
B) krypton.
C) potassium.
D) beryllium.
E) boron.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which group of the periodic table of elements forms only 2+ ions?
A) group 1A
B) group 2A
C) group 1B
D) group 7A
E) group 8A
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element has the following ground state electron configuration? 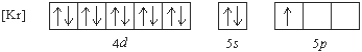
A) In
B) Y
C) Nb
D) Tl
E) Ga
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species would you expect to have the largest radius?
A) ![]()
B) P
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the maximum number of electrons that can occupy d orbital?
A) 1
B) 2
C) 6
D) 10
E) 14
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For which one of the following elements is the second ionization energy over ten times larger than its first ionization energy?
A) B
B) N
C) Li
D) Ne
E) Cu
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 82
Related Exams