A) bond 1
B) bond 2
C) bond 3
D) bond 4
E) bond 5
Correct Answer

verified
E
Correct Answer
verified
Short Answer
Ar,K+,Cl- are isoelectronic elements (elements with the same number of electrons).What orbital does the last electron occupy?
Correct Answer

verified
Correct Answer
verified
Essay
Draw the shape of a 2p orbital.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the number of pi bonds in CH3CN.
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Essay
Draw condensed structures for the four compounds with formula C3H9N.
Correct Answer

verified
CH3CH2CH2NH2 C...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
Covalent bonds may be polar or nonpolar.What property of the atoms forming a given bond determines this?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the electronic configuration of the element Fe?
A) 1s2 2s2 2p6 3s2 3p6 4s2 3d6
B) 1s2 2s2 2p6 3s2 3p8 3d6
C) 1s2 2s2 2p8 3s2 3p6 4s2 3d6
D) 1s2 2s2 2p6 3s2 3p6 4s2 4d6
E) 1s2 2s2 2p6 3s2 3p6 4s2 4p6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has the smallest dipole moment?
A) Br2
B) NH3
C) HCl
D) HBr
E) HI
Correct Answer

verified
Correct Answer
verified
Essay
Draw a Lewis structure for the molecule given and show all formal charges. CH2CO
Correct Answer

verified
Correct Answer
verified
Essay
The Kekulé structure of pentane is shown below.Draw the condensed structural formula which corresponds to this Lewis structure. 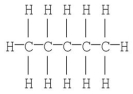
Correct Answer

verified
Correct Answer
verified
Essay
Using the symbol δ+ and δ-,show the direction of the polarity in the indicated bond. 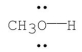
Correct Answer

verified
11ea8a1c_2c5f_9396_b657_dfc244e9ba29_TB1831_00
Correct Answer
verified
Multiple Choice
Identify the least electronegative atom.
A) P
B) Na
C) I
D) B
E) O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the number of nonbonding lone pairs of electrons in H2NOH.
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the CNN bond angle in the compound shown below? 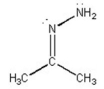
A) ~60°
B) ~90°
C) ~110°
D) ~120°
E) ~180°
Correct Answer

verified
Correct Answer
verified
Essay
Why is the C-H bond in ethene (H2C  CH2)shorter and stronger than the C-H bond in ethane (CH3CH3)?
CH2)shorter and stronger than the C-H bond in ethane (CH3CH3)?
Correct Answer

verified
The length and strength of a C-H bond depends on the hybridization of the carbon atom.The more s character in the hybrid orbital used by carbon to form the bond,the shorter and stronger the bond.This is because an s orbital is closer to the nucleus than is a p.Ethene uses carbon sp2 hybrid orbitals (1/3 s character)to make its carbon-hydrogen bonds while ethane uses carbon sp3 (1/4 s character).
Correct Answer
verified
Multiple Choice
Give the shape of the methyl radical.
A) trigonal pyramidal
B) tetrahedral
C) bent
D) linear
E) trigonal planar
Correct Answer

verified
Correct Answer
verified
Essay
BF3 has a dipole moment of zero.Propose a structure for BF3 that is consistent with this information.
Correct Answer

verified
BF3 is trig...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Identify the hybridization of the oxygen in CH3OCH3.
A) sp
B) sp2
C) sp3
D) sp4
E) sp5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an sp2 hybridized carbon?
A) ![]()
B) ∙ CH3
C) ![]()
D) A and B
E) A,B and C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many unpaired electrons are present in the isolated carbon atom (atomic number = 6) ?
A) none
B) one
C) two
D) three
E) four
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 90
Related Exams