A) an acyclic β-diketone
B) an acyclic β-ketoacid
C) an acyclic β-diester
D) a cyclic β-ketoester
E) a cyclic β-diketone
Correct Answer

verified
Correct Answer
verified
Essay
Provide the structure of the Claisen product in the self-condensation of methyl phenylacetate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When compared to the keto form,the enol form of which of the following compounds is most stable? 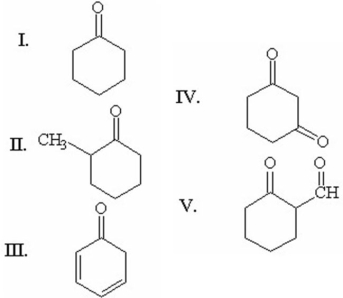
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Essay
Provide the major organic product of the following. 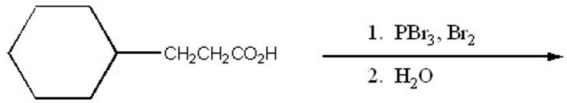
Correct Answer

verified
Correct Answer
verified
Essay
When compound X is heated,PhCOCH(CH3)2 and CO2 are produced.Offer a structure for compound X.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A student proposed the following synthesis.Why did it fail? 
A) The use of LDA results in the thermodynamic product instead of the desired kinetic product.
B) LDA is insoluble in the THF solvent so the reaction is too slow to occur at a useful rate.
C) LDA acts as a nucleophile instead of a base in its reaction with cyclohexanone.
D) The tertiary bromide is too sterically hindered to be attacked by the enolate.
E) Instead of 2-methyl-2-bromopentane,2-bromo-3-methylpentane should have been used to anticipate a cationic rearrangement that would occur.
Correct Answer

verified
Correct Answer
verified
Essay
Use an asterisk to indicate the most electron-rich carbon in the molecule below. 
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the major product for the following reaction. 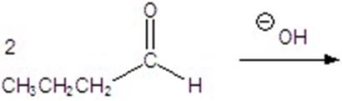
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the labeled hydrogen atoms in the following structure is the most acidic? 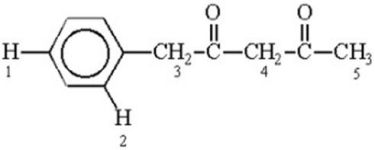
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Essay
Provide a detailed,stepwise mechanism for the formation of acetate and bromodiiodomethane from bromoacetone,hydroxide and iodine.
Correct Answer

verified
Correct Answer
verified
Essay
Fill in the empty boxes. 
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the major product for the reaction. 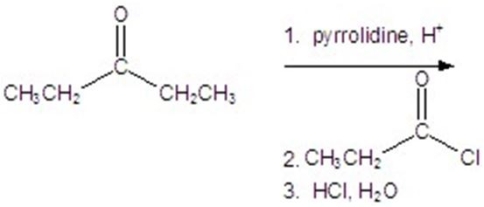
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Hell-Volhard-Zelinsky reaction involves
A) the α-bromination of carboxylic acids.
B) the α-bromination of ketones.
C) the bromination of alcohols.
D) the oxidation of aldehydes to acids.
E) none of the above.
Correct Answer

verified
Correct Answer
verified
Essay
Give the major product for the following reaction. 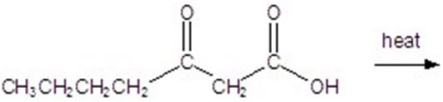
Correct Answer

verified
Correct Answer
verified
Essay
Explain why the following acid cannot be prepared by the malonic ester synthesis. 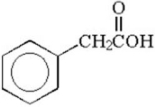
Correct Answer

verified
Since bromobenzene is needed to prepare ...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Provide a detailed,stepwise mechanism for the α-bromination of acetone in base.
Correct Answer

verified
Correct Answer
verified
Essay
Give the major product for the following reaction. 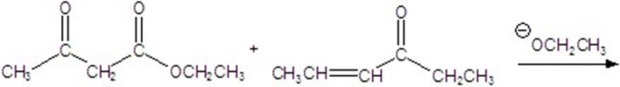
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What materials are needed to prepare the following product? 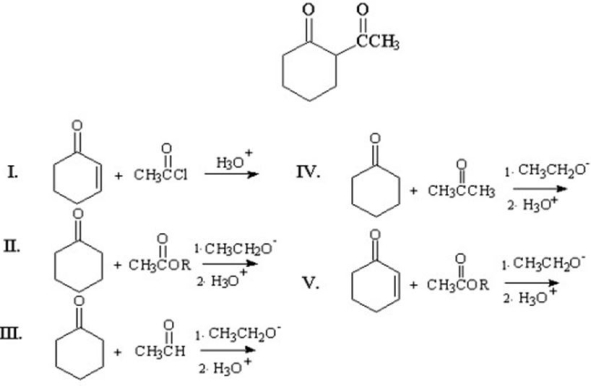
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Essay
Provide the major organic product(s)of the reaction shown below. 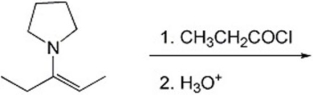
Correct Answer

verified
Correct Answer
verified
Essay
Provide the major organic product of the following reaction. 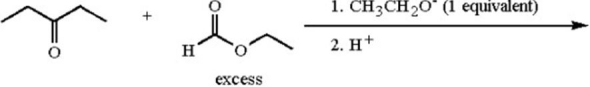
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 121
Related Exams