The isotopes 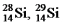 And
And 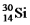 Are all stable,while
Are all stable,while 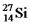 Is radioactive.The mode of decay for
Is radioactive.The mode of decay for 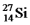 Is most likely to be
Is most likely to be
A) positron decay.
B) alpha decay.
C) beta decay.
D) gamma decay.
E) fission.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
What is the specific activity (in Ci/g) of an isotope if 3.56 mg emits 4.26 108 particles per second?
A) 0.003232 Ci/g
B) 0.0115 Ci/g
C) 0.309 Ci/g
D) 3.23 Ci/g
E) None of these choices is correct.
G) A) and C)
Correct Answer

verified
Correct Answer
verified