A) 3.94 × ![]()
M
B) 2.54 × ![]()
M
C) 1.0 × ![]()
M
D) not enough information
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
A solution with a concentration of 0.000 000 1 M HCl would be an acidic solution.
Correct Answer

verified
Correct Answer
verified
True/False
The main component of vinegar is acetic acid.
Correct Answer

verified
Correct Answer
verified
True/False
The burning of fossil fuels produces nitrogen oxides and sulfur oxides which can react to form acid rain (nitric and sulfuric acids).
Correct Answer

verified
True
Correct Answer
verified
Multiple Choice
Which combination below will be a buffer solution?
A) HCl and Cl⁻
B) HNO3 and NaNO3
C) H ![]()
![]()
![]()
And Na
![]()
![]()
![]()
D) NaBr and NaOH
E) All of the above are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the [OH-] in a solution that has a pOH of 9.65?
A) 4.5 × ![]()
M
B) 4.5 × ![]()
M
C) 9.8 × ![]()
M
D) 2.2 × ![]()
M
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following acids is diprotic?
A) HCl ![]()
B) HN ![]()
C) HI
D) ![]()
![]()
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The ion product constant for water (Kw)is best stated as Kw = [H2O] [H+].
Correct Answer

verified
Correct Answer
verified
True/False
Since diprotic acids such as H2CO3 have two ionizable protons,they will always behave as strong acids compared to monoprotic acids.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 35.0 mL sample of 0.225 M HBr was titrated with 42.3 mL of KOH.What is the concentration of the KOH?
A) 0.157 M
B) 0.303 M
C) 0.272 M
D) 0.186 M
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
A strong acid is one that completely dissociates into ions in solution.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the concentration of 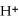 In a solution with pH = 2.50?
In a solution with pH = 2.50?
A) 4.5 M
B) 1.3 M
C) 0.0032 M
D) 0.40 M
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In order for a solution to be basic:
A) [H3O+] > [OH-]
B) [H3O+] < [OH-]
C) [H3O+] = [OH-]
D) pH = pOH
E) none of the above
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following acids is commonly used for manufacturing fertilizer?
A) HCl
B) HN ![]()
C) HF
D) HBr
E) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements are TRUE of buffer solutions? 1.A buffer solution can be made by mixing equal concentrations of Acetic acid and sodium acetate. 2.A buffer solution can be made by mixing equal concentrations of Hydrochloric acid and sodium chloride. 3.A buffer solution resists changes in pH when small quantities of acid Or base are added.
A) 1 and 2 only
B) 2 and 3 only
C) 1 and 3 only
D) All of 1,2,and 3
E) None of 1,2,and 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the concentration of the hydronium ions in an acidic solution?
A) 0.0 M
B) 1.0 ![]()
M
C) 1.0 ![]()
M
D) > 1.0 ![]()
M
E) < 1.0 ![]()
M
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
A 0.10 M solution of an electrolyte has a pH of 4.5.The electrolyte is:
A) a strong acid.
B) a strong base.
C) a weak acid.
D) a weak base.
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Water always acts as an acid in reactions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the conjugate acid of OH⁻?
A) ![]()
B) ![]()
O
C) NaOH
D) OH⁻
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The pH of 0.001 M HCl is 3.0.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 117
Related Exams