Which of the following atomic symbols is incorrect?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and D)
Correct Answer

verified
Correct Answer
verified
The numbers of protons,neutrons,and electrons in 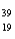 K+ are:
K+ are:
A) 20 p,19 n,19 e
B) 20 p,19 n,20 e
C) 19 p,20 n,20 e
D) 19 p,20 n,19 e
E) 19 p,20 n,18 e
G) C) and D)
Correct Answer

verified
Correct Answer
verified