A) large differences in elemental composition from organism to organism.
B) differences in the types and relative amounts of organic molecules synthesized by each organism.
C) differences in the elements that bond with carbon in each organism.
D) differences in the sizes of the organic molecules in each organism.
E) differences in the inorganic compounds present in each organism.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The complexity and variety of organic molecules is due to
A) the chemical versatility of carbon atoms.
B) the variety of rare elements in organic molecules.
C) the fact that they can be synthesized only in living organisms.
D) their interaction with water.
E) their tremendously large sizes.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
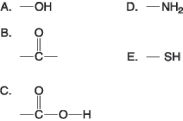 Figure 3.4
-Which of the groups shown in Figure 3.4 is a functional group that helps stabilize proteins by forming covalent cross-links within or between protein molecules?
Figure 3.4
-Which of the groups shown in Figure 3.4 is a functional group that helps stabilize proteins by forming covalent cross-links within or between protein molecules?
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound contains hydroxyl groups as its predominant functional group. Which of the following statements is true concerning this compound?
A) It lacks an asymmetric carbon, and it is probably a fat or lipid.
B) It should dissolve in water.
C) It should dissolve in a nonpolar solvent.
D) It won't form hydrogen bonds with water.
E) It is hydrophobic.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
The molecular formula for glucose is C6H12O6. What would be the molecular formula for a polymer made by linking ten glucose molecules together by dehydration reactions?
A) C60H120O60
B) C6H12O6
C) C60H102O51
D) C60H100O50
E) C60H111O51
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 14C-labeled uracil is added to the growth medium of cells, what macromolecules will be labeled?
A) phospholipids
B) DNA
C) RNA
D) both DNA and RNA
E) proteins
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning saturated fats is not true?
A) They are more common in animals than in plants.
B) They have multiple double bonds in the carbon chains of their fatty acids.
C) They generally solidify at room temperature.
D) They contain more hydrogen than unsaturated fats having the same number of carbon atoms.
E) They are one of several factors that contribute to atherosclerosis.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Dehydration reactions are used in forming which of the following compounds?
A) triacylglycerides
B) polysaccharides
C) proteins
D) triacylglycerides and proteins only
E) triacylglycerides, polysaccharides, and proteins
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which chemical group is most likely to be responsible for an organic molecule behaving as a base (see Concept 2.5) ?
A) hydroxyl
B) carbonyl
C) carboxyl
D) amino
E) phosphate
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Testosterone and estradiol are male and female sex hormones, respectively, in many vertebrates. In what way(s) do these molecules differ from each other?
A) Testosterone and estradiol are structural isomers but have the same molecular formula.
B) Testosterone and estradiol are cis-trans isomers but have the same molecular formula.
C) Testosterone and estradiol have different functional groups attached to the same carbon skeleton.
D) Testosterone and estradiol have distinctly different chemical structures, with one including four fused rings of carbon atoms, whereas the other has three rings.
E) Testosterone and estradiol are enantiomers of the same organic molecule.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which type of interaction stabilizes the α helix and the β pleated sheet structures of proteins?
A) hydrophobic interactions
B) disulfide bonds
C) ionic bonds
D) hydrogen bonds
E) peptide bonds
Correct Answer

verified
Correct Answer
verified
Multiple Choice
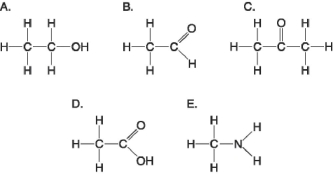 Figure 3.5
-Which molecule shown in Figure 3.5 would have a positive charge in a cell?
Figure 3.5
-Which molecule shown in Figure 3.5 would have a positive charge in a cell?
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
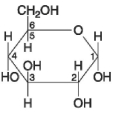 Figure 3.7
-Which of the following descriptors is true of the molecule shown in Figure 3.7?
Figure 3.7
-Which of the following descriptors is true of the molecule shown in Figure 3.7?
A) hexose
B) fructose
C) glucose
D) hexose and fructose only
E) hexose and glucose only
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
Which of the following is not a polymer?
A) glucose
B) starch
C) cellulose
D) chitin
E) DNA
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these molecules is not formed by dehydration reactions?
A) fatty acids
B) disaccharides
C) DNA
D) protein
E) amylose
Correct Answer

verified
Correct Answer
verified
Multiple Choice
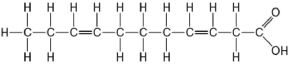 Figure 3.9
-Which of the following statements is true regarding the molecule illustrated in Figure 3.9?
Figure 3.9
-Which of the following statements is true regarding the molecule illustrated in Figure 3.9?
A) It is a saturated fatty acid.
B) A diet rich in this molecule may contribute to atherosclerosis.
C) Molecules of this type are usually liquid at room temperature.
D) It is a saturated fatty acid, and a diet rich in this molecule may contribute to atherosclerosis.
E) It is a saturated fatty acid, a diet rich in this molecule may contribute to atherosclerosis, and molecules of this type are usually liquid at room temperature.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemist wishes to make an organic molecule less acidic. Which of the following functional groups should be added to the molecule in order to do so?
A) carboxyl
B) sulfhydryl
C) hydroxyl
D) amino
E) phosphate
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
On food packages, to what does the term insoluble fiber refer?
A) cellulose
B) polypeptides
C) starch
D) amylopectin
E) chitin
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The structural level of a protein least affected by a disruption in hydrogen bonding is the
A) primary level.
B) secondary level.
C) tertiary level.
D) quaternary level.
E) All structural levels are equally affected.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the structural feature that allows DNA to replicate?
A) sugar-phosphate backbone
B) complementary pairing of the nitrogenous bases
C) disulfide bonding (bridging) of the two helixes
D) twisting of the molecule to form an α helix
E) three-component structure of the nucleotides
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 121
Related Exams