Correct Answer

verified
is the wavelength of a ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
The 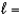 3 subshell contains how many total electrons?
3 subshell contains how many total electrons?
A) 2
B) 6
C) 10
D) 14
E) 18
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What color will a blue object appear when it is seen through a filter with the absorption spectrum shown below? 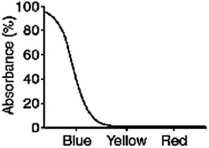
A) blue
B) yellow
C) red
D) black
E) white
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents a p orbital?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which arrangement is correct for increasing atomic radius?
A) C ![]() N
N ![]() F
F
B) Rb ![]() K
K ![]() Li
Li
C) Sc ![]() Ge
Ge ![]() Br
Br
D) Se ![]() Cu
Cu ![]() Ca
Ca
E) H ![]() He
He
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electron configuration of a Cl ion?
A) 1s22s22p63s23p4
B) 1s22s22p63s23p5
C) 1s22s22p63s23p6
D) 1s22s22p63s23p2
E) 1s22s22p63s23p8
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the energy (E, in J) of a photon from a microwave oven with a frequency of 6.0 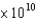 Hz?
Hz?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A certain shell is known to have a total of 9 orbitals. Which shell is it?
A) n ![]() 1
1
B) n ![]() 2
2
C) n ![]() 3
3
D) n ![]() 4
4
E) n ![]() 5
5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the minimum frequency of a photon that can eject a photoelectron from Ba metal? (The work function of barium is 4.3 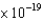 J.)
J.)
A) 2.8 ![]() Hz
Hz
B) 6.5 ![]() Hz
Hz
C) 6.5 ![]() Hz
Hz
D) 6.5 ![]() Hz
Hz
E) 2.8 ![]() Hz
Hz
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What 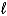 quantum numbers are possible when n 3?
quantum numbers are possible when n 3?
A) 1 and 2
B) 1, 2, and 3
C) 0, 1, 2, and 3
D) 0, 1, and 2
E) any integer
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which arrangement is not the correct order of increasing first ionization energy?
A) Li < C < F
B) Rb < K < Na
C) I < Br < F
D) Si < S < Ar
E) Be < Mg < Ca
Correct Answer

verified
Correct Answer
verified
Short Answer
Recently, buckyballs (C60) became the largest objects with a measured de Broglie wavelength. If the mass of a C60 molecule is 1.20 1024 kg, what will be its de Broglie wavelength if it is moving at a speed of 220 m/sec?
Correct Answer

verified
Correct Answer
verified
Essay
Write the electron configuration for each of the following atoms: a) Zn b) C c) P d) Sr.
Correct Answer

verified
a) [Ar]3d104...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which statement about the quantum numbers that identify an atomic orbital is not correct?
A) The principal quantum number, n, identifies the size of an atomic orbital.
B) The angular momentum quantum number, ![]() , identifies the shape of an atomic orbital.
, identifies the shape of an atomic orbital.
C) The magnetic quantum number, ml, identifies the orientation of the orbital in space.
D) The magnetic quantum number can have values that range from ![]() to
to ![]() in integer steps.
in integer steps.
E) The principal quantum number alone, n, identifies the energy of an atomic orbital.
Correct Answer

verified
Correct Answer
verified
Essay
When the principle quantum number 3, what values are possible for the other quantum numbers 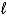 and m
and m 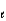 ?
?
Correct Answer

verified
 can equal 0, 1, and 2.
can equal 0, 1, and 2.
For  0, m
0, m 
can ...View Answer
Show Answer
Correct Answer
verified
For
can ...
View Answer
Multiple Choice
What is the orbital designation for an electron with the quantum numbers n  4,
4, 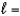 3?
3?
A) 4s
B) 4p
C) 4d
D) 4f
E) cannot determine from the given information
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many orbitals exist for the quantum numbers n  4,
4, 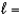 1?
1?
A) 1
B) 2
C) 3
D) 4
E) 7
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What ml quantum numbers are possible when n =2 and I=1?
A) 0 and 1
B) (1, 0, and 1)
C) 0, 1 and, 2
D) (2, 1, 0, 1, and 2)
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many unpaired electrons does the nitride (N3) ion have?
A) 0
B) 1
C) 2
D) 3
E) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following electron configurations represents an excited state?
A) [Ne]3s23p5
B) [Kr]4d105s25p1
C) [Ar]3d104s24p6
D) [Ne]3s23p64s1
E) [Kr]4d105s15p1
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 166
Related Exams