A) 0.00 M. All of the silver ions are used up to make the precipitate.
B) 0.100 M. The silver ions do not participate in the chemical reaction and are considered spectator ions.
C) greater than 0.100 M. For every 1.0 mol of silver nitrate there are 2.0 mol of silver ions in solution.
D) less than 0.100 M. Even though silver ions do not react, they are diluted when mixed with the sodium chloride solution.
E) less than 0.100 M. Some, but not all, of the silver ions are used to make the precipitate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution is prepared by dissolving 8.29 g of Na2SO4 in enough water to make 225 mL of solution. Calculate the solution molarity.
A) 0.0584 M
B) 1.87 M
C) 0.603 M
D) 0.259 M
E) 0.484 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following solutions contains the smallest number of ions?
A) 539.8 mL of 1.0 M lithium nitrate
B) 305.6 mL of 2.0 M potassium hydroxide
C) 189.6 mL of 2.0 M iron(III) chloride
D) 246.8 mL of 1.0 M sodium sulfate
E) At least two of the above contain the smallest number of ions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You have two HCl solutions, labeled solution A and solution B. Solution A has a greater concentration than solution B. Which of the following statements is/are true?
A) If you have equal volumes of both solutions, solution B must contain more moles of HCl.
B) If you have equal moles of HCl in both solutions, solution B must have a greater volume.
C) To obtain equal concentrations of both solutions, you must add a certain amount of water to solution B.
D) Adding more moles of HCl to both solutions will make them less concentrated.
E) At least two of the above statements are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 27.9-g sample of HF is dissolved in enough water to give 2.0 * 102 mL of solution. The concentration of the solution is
A) 27.9 M
B) 1.39 M
C) 0.28 M
D) 7.0 M
E) 5.58 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the normality of a base if 93.0 mL of 1.8 M HCl is required to neutralize 52.0 mL of it.
A) 1.01 N
B) 0.31 N
C) 1.0 N
D) 94 N
E) 3.2 N
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What mass of calcium chloride, CaCl2, is needed to prepare 2.657 L of a 1.56 M solution?
A) 4.14 g
B) 313 g
C) 189 g
D) 65.2 g
E) 460 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume of 18.0 M sulfuric acid is required to prepare 26.2 L of 0.126 M H2SO4?
A) 5.45 mL
B) 0.183 L
C) 472 mL
D) 3.30 L
E) 208 mL
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a 74.6-g sample of ammonium nitrate is dissolved in enough water to make 315 mL of solution, what will be the molarity?
A) 0.932 M
B) 2.96 M
C) 0.294 M
D) 3.41 M
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you mix 40.0 mL of a 0.389 M solution of K2CrO4 with 40.0 mL of a 0.389 M solution of AgNO3, what mass of solid forms?
A) 5.16 g
B) 2.58 g
C) 3.02 g
D) 15.6 g
E) 7.78 g
Correct Answer

verified
Correct Answer
verified
True/False
Approximately 38 g of NaCl can be dissolved in 100 g of water at 25°C. A solution prepared by adding 35 g of NaCl to 100 g of water at 25°C is unsaturated.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You have 3.00 L of a 3.48 M solution of NaCl(aq) called solution A. You also have 2.00 L of a 2.00 M solution of AgNO3(aq) called solution B. You mix these solutions together, making solution C. Calculate the concentration (in M) of Na+ ions in solution C.
A) 0 M
B) 3.48 M
C) 5.22 M
D) 1.10 M
E) 2.09 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following acid-base neutralization, 1.61 g of the solid acid HC6H5O neutralized 11.61 mL of aqueous NaOH solution base by the reaction NaOH(aq) + HC6H5O(aq) H2O(l) + NaC6H5O(aq) Calculate the molarity of the base solution.
A) 1.47 M
B) 17.1 M
C) 7.21 M
D) 0.139 M
E) 0.199 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemist needs 225 mL of 3.1 M HCl. What volume of 12 M HCl must be dissolved in water to form this solution? (Ignore significant figures for this problem.)
A) ![]() mL
mL
B) 15 mL
C) 6.0 mL
D) 17 mL
E) 58 mL
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You mix 100.0 mL of a 0.100 M NaOH solution and 150.0 mL of a 0.104 M HCl solution. Determine the concentration of H+ in the final mixture after the reaction is complete.
A) 0.0624 M
B) 0.104 M
C) 0.156 M
D) 0.204 M
E) 0.0224 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 0.216-g sample of NaCl (molar mass = 58.44 g/mol) is dissolved in enough water to make 5.20 mL of solution. Calculate the molarity of the resulting solution.
A) 0.711 M
B) ![]() M
M
C) 0.823 M
D) 1.41 M
E) 0.567 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An oven-cleaning solution is 40.0% (by mass) NaOH. If one jar of this product contains 457.0 g of solution, how much NaOH does it contain?
A) ![]() g
g
B) 11.4 g
C) 183 g
D) 18.3 g
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You have 3.00 L of a 3.67 M solution of NaCl(aq) called solution A. You also have 2.00 L of a 2.00 M solution of AgNO3(aq) called solution B. You mix these solutions together, making solution C. Calculate the concentration (in M) of Ag+ ions in solution C.
A) 0 M
B) 1.40 M
C) 2.20 M
D) 0.800 M
E) 3.51 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You have three sodium carbonate solutions on a lab table in front of you. All of the solutions came from a 500.0-mL volumetric flask containing 3.00 M sodium carbonate. Solution 1 contains 100.0 mL of the 3.00 M solution. Solution 2 contains 50.0 mL of the 3.00 M solution. Solution 3 contains 10.0 mL of the 3.00 M solution. What volume of water (in mL) must evaporate from Solution 1 in order to have a concentration of 4.48 M?
A) 67.0 mL
B) 49.3 mL
C) 33.0 mL
D) 14.9 mL
E) 85.1 mL
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You have 3.00 L of a 3.00 M solution of NaCl(aq) called solution A. You also have 2.00 L of a 1.84 M solution of AgNO3(aq) called solution B. You mix these solutions together, making solution C. Calculate the concentration (in M) of 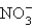 ions in solution C.
ions in solution C.
A) 0 M
B) 0.736 M
C) 1.06 M
D) 1.84 M
E) 1.80 M
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 70
Related Exams