A) C
B) N
C) O
D) P
E) S
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which energy sublevel is being filled by the elements Rb to Sr?
A) 4d
B) 5s
C) 5p
D) 5d
E) 5f
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following groups has a predictable ionic charge of two negative?
A) Group IIA/2
B) Group IIB/12
C) Group VIA/16
D) Group VIB/6
E) Group VIIIA/18
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements has the highest ionization energy?
A) Cl
B) Br
C) Ar
D) Kr
E) K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the predicted melting point for tungsten metal,W,given the melting points of chromium,Cr, (1857 °C) and molybdenum,Mo, (2617 °C) .
A) 760 °C
B) 1097 °C
C) 2237 °C
D) 2997 °C
E) 3377 °C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the lowest atomic mass lanthanide?
A) Ce
B) Lr
C) Lu
D) Sc
E) Th
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the number of valence electrons for a Group VIIA/17 element.
A) 1
B) 2
C) 3
D) 7
E) 17
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a radioactive metal?
A) Pb
B) Pd
C) Pm
D) Pt
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has chemical properties most similar to sodium?
A) He
B) K
C) Mg
D) B
E) Fe
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the electron dot formula for an atom of carbon? 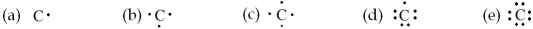
A) (a)
B) (b)
C) (c)
D) (d)
E) (e)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a solid semimetal at normal conditions?
A) Al
B) Ge
C) P
D) all of the above
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the predicted density for rhodium,Rh,given the density of cobalt,Co, (8.89 g/cm3) and iridium,Ir, (22.65 g/cm3) .
A) 2.01 g/cm3
B) 6.88 g/cm3
C) 13.76 g/cm3
D) 15.77 g/cm3
E) 29.53 g/cm3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ions is not isoelectronic with the noble gas krypton?
A) Ga3+
B) Se2-
C) Br-
D) Sr2+
E) Zr4+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a noble gas?
A) F
B) N
C) O
D) all of the above
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a halogen?
A) Ar
B) Br
C) H
D) Hg
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the electron configuration using core notation for Sr2+?
A) [Kr]
B) [Kr] 5S₂
C) [Kr] 4d10
D) [Kr] 5S₂ 4d8
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which fifth period transition element has the highest atomic number?
A) Cd
B) Hg
C) Sr
D) Xe
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the term for the elements in Groups 3-12?
A) rare earth elements
B) representative elements
C) transition elements
D) inner transition elements
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the electron configuration using core notation for Se2-?
A) [Ar] 4S₂ 3d10 4p2
B) [Ar] 4S₂ 3d10 4p4
C) [Ar] 4S₂ 4p6
D) [Kr]
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the core notation for the electron configuration of a potassium atom?
A) [Ar]
B) [Ar] 4s1
C) [Ar] 4p1
D) [Ar] 4d1
E) [Kr]
Correct Answer

verified
Correct Answer
verified
Showing 121 - 140 of 209
Related Exams