A) the most stable product.
B) the product that can be formed in the fewest steps.
C) the product whose formation requires the smallest free energy of activation.
D) the product with the greatest potential energy.
E) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons does the HOMO of 2,4-hexadiene have in its ground state?
A) 1
B) 2
C) 3
D) 4
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true of pericyclic reactions?
A) they are concerted reactions
B) proceed via a cyclic transition state
C) no intermediates are formed
D) all of these
Correct Answer

verified
Correct Answer
verified
Short Answer
Use Woodward-Fieser rules to estimate the max for the following compound. 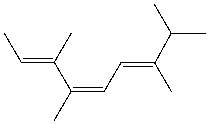
Correct Answer

verified
Correct Answer
verified
Short Answer
Which of the following compounds have isolated double bonds? 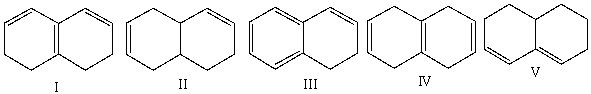
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is (are) a isolated diene(s) ?
A) 4-methyl-1,3-heptadiene
B) 3-methyl-1,5-heptadiene
C) 2-methyl-2,4-heptadiene
D) 4-methyl-1,4-heptadiene
E) 5-methyl-2,3-heptadiene
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following diene(s) cannot undergo the Diels-Alder reaction? 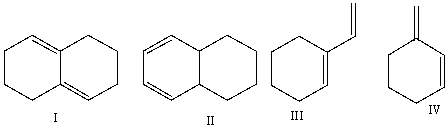
A) I
B) II
C) III
D) IV
E) I & IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best describes the stereochemistry of ring closure and the product for the following reaction? 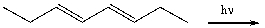
A) disrotatory, cis-3,4-diethylcyclobutene
B) conrotatory, cis-3,4-diethylcyclobutene
C) disrotatory, trans-3,4-diethylcyclobutene
D) conrotatory, trans -3,4-diethylcyclobutene
Correct Answer

verified
Correct Answer
verified
Essay
Predict the product(s) for the following reaction. 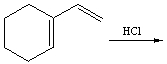
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following dienes is most stable? 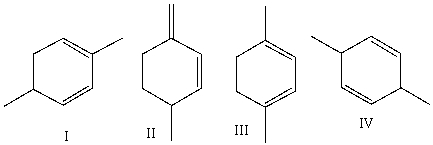
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Essay
Predict the major product for the following intramolecular Diels-Alder reaction and provide a curved arrow mechanism for the formation of the product. 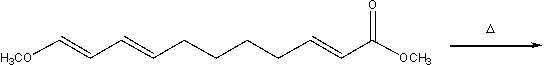
Correct Answer

verified
Correct Answer
verified
Essay
Predict the product for the following reaction and provide the curved arrow mechanism for formation of the product. 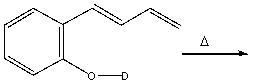
Correct Answer

verified
Correct Answer
verified
Essay
Predict the product for the following Diels-Alder reaction. 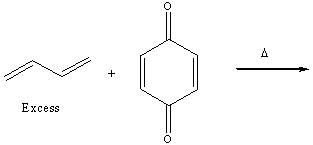
Correct Answer

verified
Correct Answer
verified
Essay
Predict the product for the following reaction. 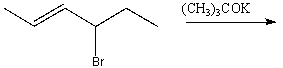
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name for the following compound? 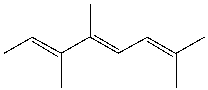
A) (2E,4Z,6E) -3,4,7,8-tetramethyl-2,4,6-heptatriene
B) (2Z,4E) -3,4,7-trimethyl-2,4,6-octatriene
C) (2E,4Z,6E) -2,5,6,7-tetramethyl-3,5,7-heptatriene
D) (2E,4Z) - 2,5,6-trimethyl-3,5,7-octatriene
E) (4E,6E) -2,5,6-trimethyl-2,4,6-octatriene
Correct Answer

verified
Correct Answer
verified
Essay
Predict the product for the following Cope rearrangement and provide the curved arrow mechanism for formation of the product. 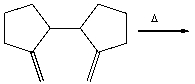
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following represents the lowest energy -bonding molecular orbital of 1,3-butadiene? 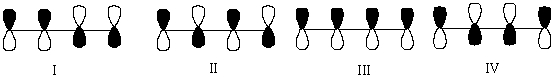
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds is not a product of reaction between 1,3-butadiene and HBr?
A) (S) -3-bromo-1-butene
B) (R) -3-bromo-1-butene
C) (E) -1-bromo-2-butene
D) (Z) -1-bromo-2-butene
E) (Z) -2-bromo-2-butene
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is (are) a conjugated diene(s) ? 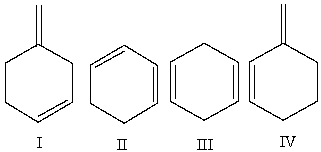
A) I
B) II
C) III
D) IV
E) II & IV
Correct Answer

verified
Correct Answer
verified
Essay
Predict the product for the following reaction. 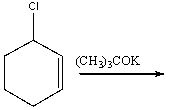
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 131
Related Exams