A) 2.94 L
B) 19.3 L
C) 32.4 L
D) 54.5 L
E) 356 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 25.5 L of oxygen are cooled from 150°C to 50°C at constant pressure,what is the new volume of oxygen?
A) 0.0514 L
B) 19.5 L
C) 33.4 L
D) 0.03 L
E) 3.5 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar mass of an unknown gas if a sample weighing 0.389 g is collected in a flask with a volume of 102 cm3 at 97°C and at a pressure of 728 mmHg? (R = 0.08206 L • atm/K • mol,1 atm = 760 mmHg) .
A) 187 g/mol
B) 121 g/mol
C) 112 g/mol
D) 31.6 g/mol
E) 8.28 × 10-3 g/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the gas with the highest average kinetic energy per mole at 298 K.
A) O2
B) CO2
C) H2O
D) H2
E) All have the same average kinetic energy.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which figure best represents the concept of pressure? (A sphere represents a gaseous atom.)
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of a gas occupies 1.40 L at 25°C and 760 mmHg.What volume will it occupy at the same temperature and 380 mmHg?
A) 2.8 L
B) 2.10 L
C) 1.40 L
D) 1.05 L
E) 0.700 L
Correct Answer

verified
Correct Answer
verified
Short Answer
A(n)___________ is an instrument used to measure the atmospheric pressure.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of gas occupies 24.5 L at a pressure of 1.57 atm.What is the pressure if the volume is increased to 48.3 L at constant temperature?
A) 0.796 atm
B) 1.26 atm
C) 3.10 atm
D) 5.38 × 10-4 atm
E) 1.86 × 103 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a substance that remains a gas under the conditions listed,deviation from the ideal gas law would be most pronounced at
A) 100°C and 2.0 atm.
B) 0°C and 2.0 atm.
C) -100°C and 2.0 atm.
D) -100°C and 4.0 atm.
E) 100°C and 4.0 atm.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 2.38 mol of a gas has a volume of 120.0 mL,what is the volume of 1.97 mol of the gas at the same temperature and pressure?
A) 25.6 mL
B) 99.3 mL
C) 119 mL
D) 145 mL
E) 563 mL
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula which describes the relationship between the pressure,volume,temperature,and moles?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the volume of NH3 produced in the following reaction when 3.0 L of N2 reacts with 4.0 L of H2 ? N2(g) + 3H2(g) → 2NH3(g)
A) 1.5 L
B) 2.7 L
C) 6.0 L
D) 7.5 L
E) 12 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula which describes the relationship between the volume and number of moles in the sample at constant pressure and constant temperature?
A) Vn = k3
B) V2 /n = k3
C) n/V2 = k3
D) V/n = k3
E) V = -k3n
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the molar mass of Freon-11 gas if a sample weighing 0.597 g occupies 100 cm3 at 95°C and 1000 mmHg (R = 0.08206 L • atm/K • mol,1 atm = 760 mmHg) .
A) 0.19 g/mol
B) 35.3 g/mol
C) 70.9 g/mol
D) 137 g/mol
E) 384 g/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A fixed quantity of gas,initially at 600ºC,undergoes a temperature change at constant pressure in a cylinder with a movable piston.What final temperature of the gas can result in the change of state depicted below? 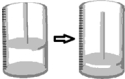
A) 1473ºC
B) 1200ºC
C) 600ºC
D) 300ºC
E) 163ºC
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the following gases in order of increasing rate of effusion: C2H6,Ar,HCl,and PH3.
A) Ar < HCl<PH3 < C2H6
B) C2H6 < PH3 < HCl < Ar
C) Ar < PH3 < C2H6 < HCl
D) C2H6 < HCl < PH3 < Ar
E) Ar < PH3 < HCl < C2H6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is false?
A) The average kinetic energies of molecules from samples of different "ideal" gases are the same at the same temperature.
B) The molecules of an ideal gas are relatively far apart.
C) All molecules of an ideal gas have the same kinetic energy at constant temperature.
D) Molecules of a gas undergo many collisions with each other and the container walls.
E) Molecules of greater mass have a lower average speed than those of less mass at the same temperature.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The catalytic decomposition of hydrogen peroxide yields oxygen gas and water.In an experiment,decomposition of hydrogen peroxide yielded 75.3 mL of gas collected over water at 25°C and 742 torr.What mass of oxygen gas was collected? (Pwater = 24 torr at 25°C,R = 0.08206 L • atm/K • mol)
A) 0.00291 g
B) 0.0931 g
C) 0.0962 g
D) 0.0993 g
E) 0.962 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
"The volume of an ideal gas is directly proportional to the number of moles of the gas at constant temperature and pressure" is a statement of _____________ law.
A) Charles's
B) Boyle's
C) Dalton's
D) Avogadro's
E) Gay-Lussac's
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of N2 gas occupies 2.40 L at 20°C.At what temperature will the N2 gas occupy a volume of 4.80 L at constant pressure?
A) 10°C
B) 40°C
C) 146°C
D) 313°C
E) 586°C
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 131
Related Exams